A recent paper uploaded to medRxiv* by Higgins et al. (2020) explored the possibility of using serologic testing as an adjunct diagnostic method in the diagnosis of SARS-CoV-2 infection.
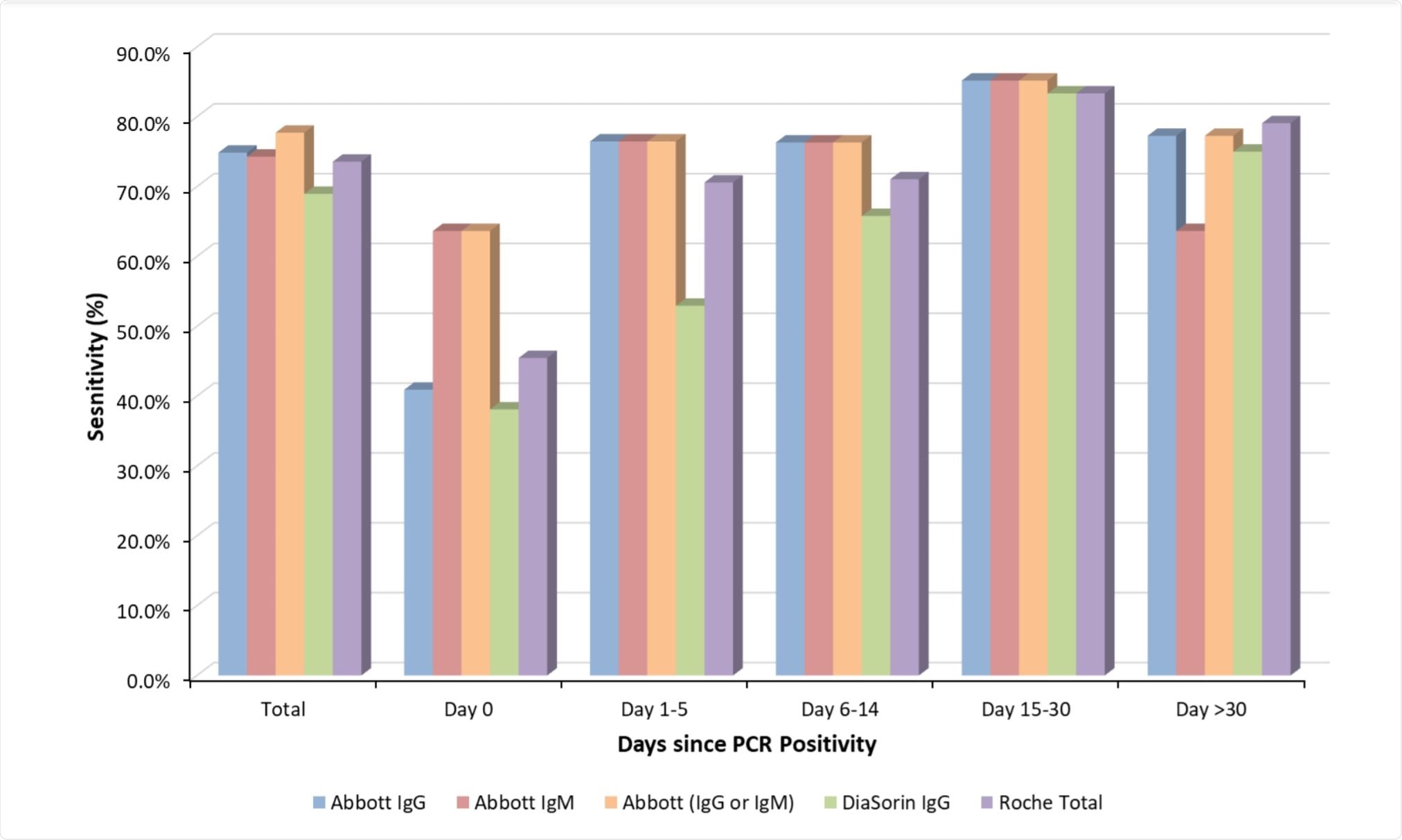
Image Credit: https://www.medrxiv.org/content/10.1101/2020.10.23.20217810v1.full.pdf

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
SARS-CoV-2 is usually confirmed in a patient by detection of viral RNA by reverse transcription-polymerase chain reaction (RT-PCR). However, the virus must be present in sufficient concentration in the nasopharyngeal/oropharyngeal sample, and this concentration can be highly variable throughout the infection.
Serologic testing is used to detect the presence and concentration of antibodies in the blood serum, and in this study, the levels of anti-SARS-CoV-2 IgA, IgG, and IgM in covid-19 diagnosed patients were determined by application to four independent commercially available anti-SARS-CoV-2 assays.
Serum and plasma were collected from 175 patients and passed through the following assays: SARS-CoV-2 IgG and SARS-CoV-2 IgM (Abbott Diagnostics); SARS-CoV-2 S1/S2 IgG (DiaSorin); and anti-SARS-CoV-2 total (Roche Diagnostics).
The first of these assays (Abbott Diagnostics) are qualitative chemiluminescent microparticle immunoassays, detecting IgG antibodies to the nucleocapsid protein of SARS-CoV-2 and IgM antibodies to the receptor-binding domain of the spike protein of SARS-CoV-2, respectively.
The second (DiaSorin) is a qualitative chemiluminescent immunoassay, detecting IgG antibodies against the spike protein of SARS-CoV-2. The final assay (Roche Diagnostics) is a qualitative chemiluminescent immunoassay that detects IgA, IgM, and IgG antibodies to the nucleocapsid protein of SARS-CoV-2.
Were the assays reliable?
A relatively wide range of antibody responses was noted throughout testing, with the average overall sensitivity ranging from 69-74.9%, and the lowest of 38-1-63.6% observed in those samples collected on the same day as RT-PCR diagnosis. All of the assays tested showed the best sensitivity more than 14 days after SARS-CoV-2 infection had been confirmed by RT-PCR, giving around an 80% confirmation rate as compared with ~58% within the first two weeks.
Each of the four assays exhibited a specificity of 100%, not detecting the presence of SARS-CoV-2 in any of the control samples from healthy individuals. Additionally, the assays were on average in agreement ~95% of the time, with the DiaSorin anti-SARS-CoV-2 IgG and Abbott anti-SARS-CoV-2 IgM assays being in agreement the least, in 89.1% of cases.
The group also examined the dynamics of anti-SARS-CoV-2 antibody concentration in five confirmed patients over 73 days, showing the great variation in immune responses between patients. IgM is often regarded as a marker of acute infection, and anti-SARS-CoV-2 IgM sensitivity has been previously identified as highest between 8-14 days after the onset of symptoms.
Similarly, anti-SARS-CoV-2 IgG assays usually provide the best sensitivity more than two weeks after the appearance of symptoms. This relationship was confirmed as a general trend in this work, and the authors suggest that three seroconversion types were observed to partially explain the heterogeneous results obtained: simultaneous IgG and IgM, IgM followed by IgG, or IgG followed by IgM.
No data was collected with regards to patient outcome or severity of symptoms, thus the group was unable to identify any correlation between the presence of particular antibodies and patient prognosis.
Other work, however, has indicated that antibody response is strongly correlated with disease severity, and so early knowledge of the rate of seroconversion following diagnosis may provide a valuable predictive tool to identify patients most at risk.
Additionally, the detection of anti-SARS-CoV-2 antibodies may also play a useful role in identifying asymptomatic patients and those who have already recovered from the infection.
The anti-SARS-CoV-2 IgM assay by Abbott Diagnostics was identified as providing the earliest and most reliable positive confirmation of SARS-CoV-2 infection within two weeks of RT-PCR confirmation, while combined testing using the anti-SARS-CoV-2 IgM and IgG assays (Abbott Diagnostics) was noted to improve sensitivity by more than 20% compared with the IgG assay alone in samples collected on the same day as RT-PCR confirmation.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
https://www.medrxiv.org/content/10.1101/2020.10.23.20217810v1.full.pdf