A paper recently uploaded to the pre-print server bioRvix* by Thomson et al. (November 2020) examines a mutation to the receptor-binding motif of the spike protein of SARS-CoV-2 that has been observed to have arisen independently twice, and is now known to be present in at least 12 countries around the world.
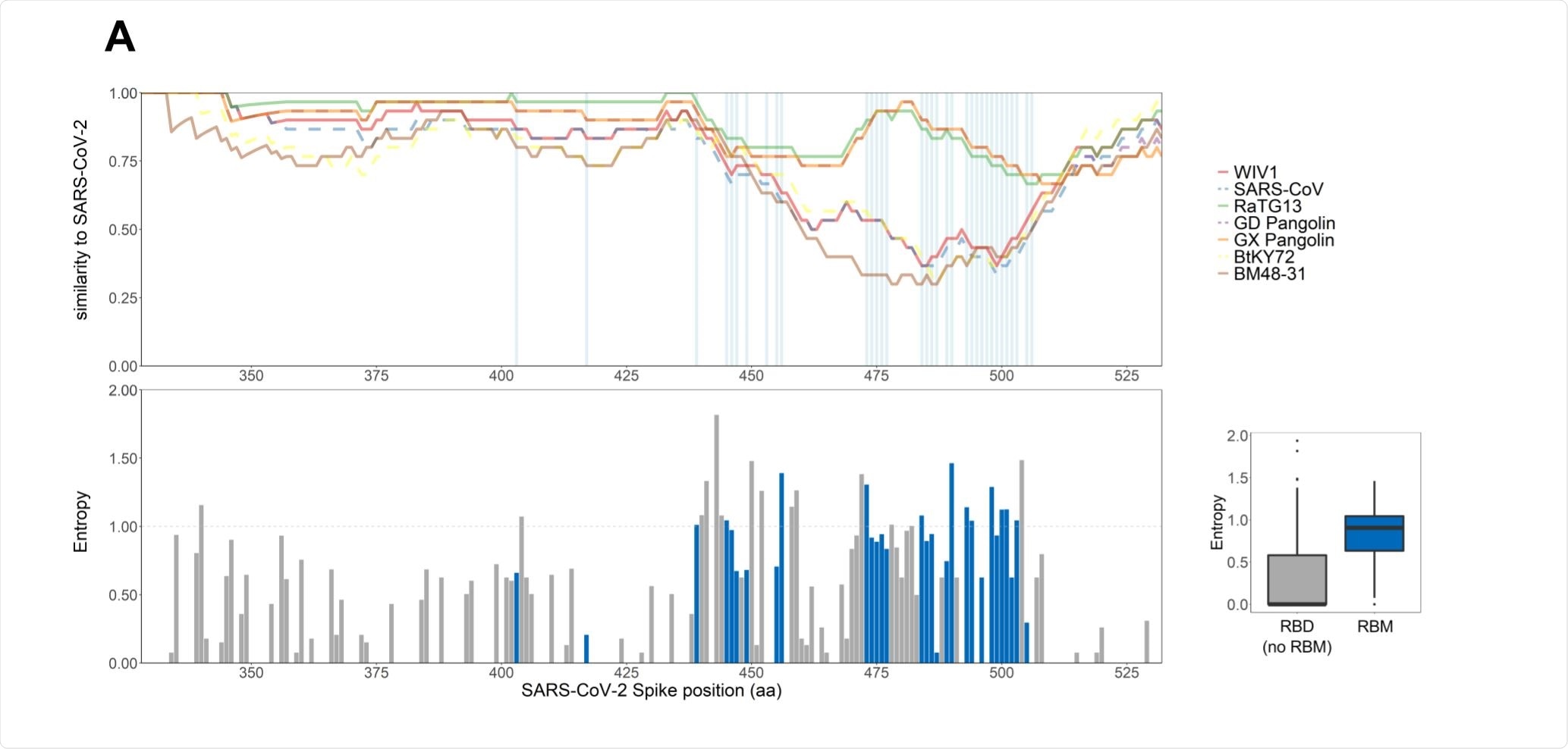
Image Credit: https://www.biorxiv.org/content/10.1101/2020.11.04.355842v1.full.pdf

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The variant, N439K, is associated with slightly higher viral loads and greater immune escape from polyclonal sera and neutralizing mAbs. This may indicate future implications for vaccine development and personalized treatment, in addition to an increased need to monitor the spread of immune escape variants of the virus.
The spike protein of SARS-CoV-2 is responsible for viral entry into the cell by its interaction with the human angiotensin-converting enzyme (hACE2) receptor and is the target of neutralizing antibodies generated by, or administered to, patients. The variant of the spike protein now dominant around the world is D614G, noted to be particularly infectious.
However, this variant may be indicated to also be particularly sensitive to neutralizing antibodies by some early studies and bears an exposed amino acid outside of the receptor-binding domain that is the target of ~90% of neutralizing antibodies in the serum of COVID-19 patients. Significant mutations to this portion of the genome may impair such treatment.
The N439K variant has increased ACE2 affinity and evades antibody-mediated immunity
As of October 2020, one of the two most commonly observed receptor binding motif (RBM) mutations is N439K, which can form an additional salt bridge with the hACE2 receptor compared to the wild type variant.
qPCR was utilized to examine the viral load of nearly 2,000 Scottish patients, finding either the N439K or N439 RBM variant in a ratio of roughly 1 to 3.7. The results suggested that the N439K variant was associated with a slightly lower cycle threshold, though did not appear to have any bearing on disease severity.
Monoclonal antibodies obtained from the serum of SARS-CoV-2 infected individuals were tested for recognition of the N439K variant, and there was noted to be a two-times reduction in the binding compared to wild type N439 in 7.4% of patients.
Interestingly, the group also evaluated a panel of 114 mAbs recovered from early SARS-CoV-2 patients that would likely only have the wild type variant. 15.5% of them had a two-fold reduction in binding to N439K, demonstrating the improved resistance of this strain.
The receptor-binding motif is highly plastic
The receptor-binding motif (RBM) mediates the entry of the virus into a host cell and is a major target of neutralizing antibodies. The group compared around 130,000 SARS-CoV-2 genomic sequences of the RBM, observing a large number of variants.
Compared to the spike protein as a whole, or the receptor-binding domain as a whole, the RBM was found to be the least conserved region. The amino acid sequences of commonly circulating viruses were tested for their binding affinity with the hACE2 receptor, and it was found that while changes to one-third of the residue could result in a notable loss of affinity, the remaining two-thirds were able to undergo a wide range of mutations without significantly affecting the ability to interact with the receptor.
The growing number of individuals with SARS-CoV-2 infection increases the likelihood of novel variants emerging, potentially damaging efforts to develop vaccines and immunotherapeutics. The development of an effective vaccine may even drive the predominance of such a strain by selective pressure.
What can be done?
The group suggests that the development of mAbs that target more highly conserved regions of SARS-CoV-2, potentially evidenced by epitopes that can target both SARS-CoV and SARS-CoV-2, and those outside of the highly plastic RBM.
An improved screening of patients to identify the presence of resistance variants would also aid in tailoring the cocktail of mAb therapeutics administered.
Image Credit: creativeneko/Shutterstock.com

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
https://www.biorxiv.org/content/10.1101/2020.11.04.355842v1