Scientists from the Oak Ridge National Laboratory at the U.S. Department of Energy headed a series of experiments that have determined that many drugs meant for treating hepatitis C can block a crucial protein enzyme, called the SARS-CoV-2 main protease, that allows reproduction in the novel coronavirus.
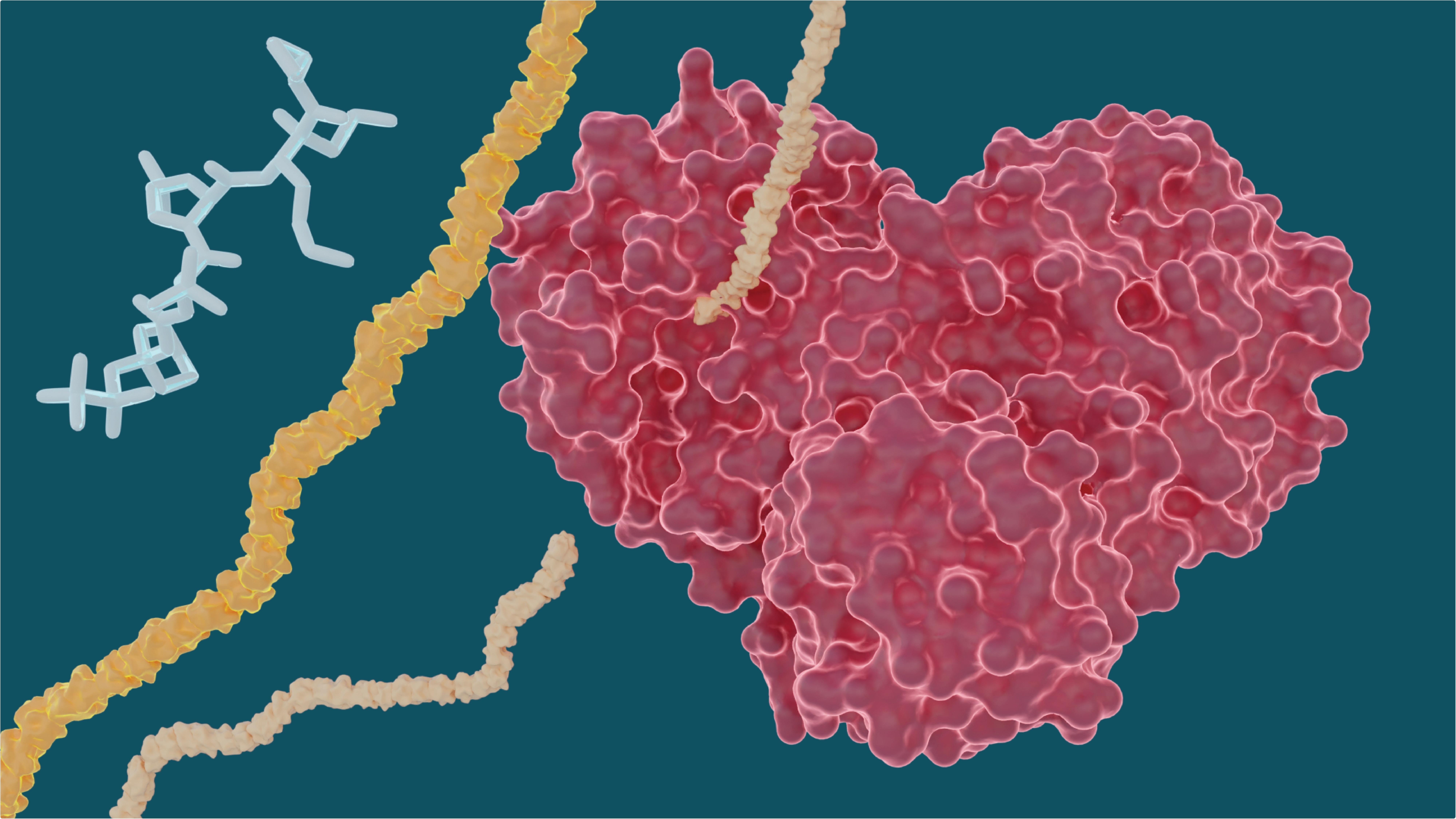
The heart-shaped SARS-CoV-2 main protease enables the virus to reproduce by cutting long chains of proteins that activate the replication process. Experiments show existing drugs used to treat hepatitis C may have the potential to treat COVID-19 by stopping the “heart” of the virus. Image Credit: Michelle Lehman, Jill Hemman/Oak Ridge National Laboratory, U.S. Department of Energy.
Blocking, or inhibiting, the function of this main protease is crucial to prevent the virus from spreading in COVID-19 patients.
Published in the Structure journal, the study is part of efforts to rapidly design pharmaceutical treatments for the COVID-19 infection by repurposing present-day medications that are known to successfully treat other kinds of diseases caused by viruses.
Currently, there are no inhibitors approved by the Food and Drug Administration that target the SARS-CoV-2 main protease. What we found is that hepatitis C drugs bind to and inhibit the coronavirus protease. This is an important first step in determining whether these drugs should be considered as potential repurposing candidates to treat COVID-19.”
Daniel Kneller, Study Lead Author, Oak Ridge National Laboratory
The SARS-CoV-2 coronavirus expresses long chains of polyproteins to spread easily. The main protease should cut these polyproteins to become functional proteins, and therefore, this protease is considered a crucial drug target by both drug developers and researchers.
In the latest research work, the investigators studied many well-known drug molecules—including a naturally occurring protease inhibitor as well as three FDA-approved hepatitis C protease inhibitors—for potential repurposing efforts.
The researchers performed X-ray measurements at room temperatures to construct a 3D map. This 3D map showed the way atoms were organized and the site where chemical bonds are formed between the drug inhibitor molecules and the protease.
The experiments produced encouraging results for specific drugs meant for treating hepatitis C and their potential to attach and block the SARS-CoV-2 main protease. Leupeptin displayed a low binding affinity and, hence, was not considered a promising candidate.
To gain a deeper understanding of how tightly or how well the inhibitors attach to the main protease, the team utilized in vitro enzyme kinetics—a method that allows scientists to analyze the inhibitor and the protease in a test tube to quantify the binding affinity, or compatibility, of the inhibitor with the protease. If the binding affinity is higher, the inhibitor will be more effective at inhibiting the function of the protease.
What we’re doing is laying the molecular foundation for these potential drug repurposing inhibitors by revealing their mode of action. We show on a molecular level how they bind, where they bind, and what they’re doing to the enzyme shape. And, with in vitro kinetics, we also know how well they bind. Each piece of information gets us one step closer to realizing how to stop the virus.”
Andrey Kovalevsky, Study Corresponding Author, Oak Ridge National Laboratory
The research work also provides a better understanding of the unusual behavior of the protease’s potential to adapt or alter its shape based on the structure and size of the inhibitor molecule it attaches to.
Pockets within the main protease, in which a drug molecule would typically bind, are extremely flexible, or malleable, and can either close or open to a certain extent based on the size of the drug molecules.
Prior to publishing the article, the investigators made their information publicly available to help and inform the medical and scientific communities. Additional studies, such as clinical trials, are required to confirm the safety and efficacy of the drugs for treating the COVID-19 disease.
The research suggests that hepatitis C inhibitors are worth thinking about as potential repurposing candidates. Immediately releasing our data allows the scientific community to start looking at the interactions between these inhibitors and the protease.”
Leighton Coates, Study Corresponding Author, Oak Ridge National Laboratory
“You can’t design a drug without knowing how it works on a molecular level, and the data we’re providing is exactly what developers need to design stronger, more tightly binding drugs for more effective treatments,” Coates concluded.
The researchers have planned to perform neutron scattering experiments to identify the positions of hydrogen atoms and the web of chemical bonds that form between the inhibitor molecules and the protease.
Source:
Journal reference:
Kneller, D. W., et al. (2020) Malleability of the SARS-CoV-2 3CL Mpro Active-Site Cavity Facilitates Binding of Clinical Antivirals. Structure. doi.org/10.1016/j.str.2020.10.007.