Within a single cell, more than a quarter of all proteins are found in the membrane, in which they carry out crucial functions.
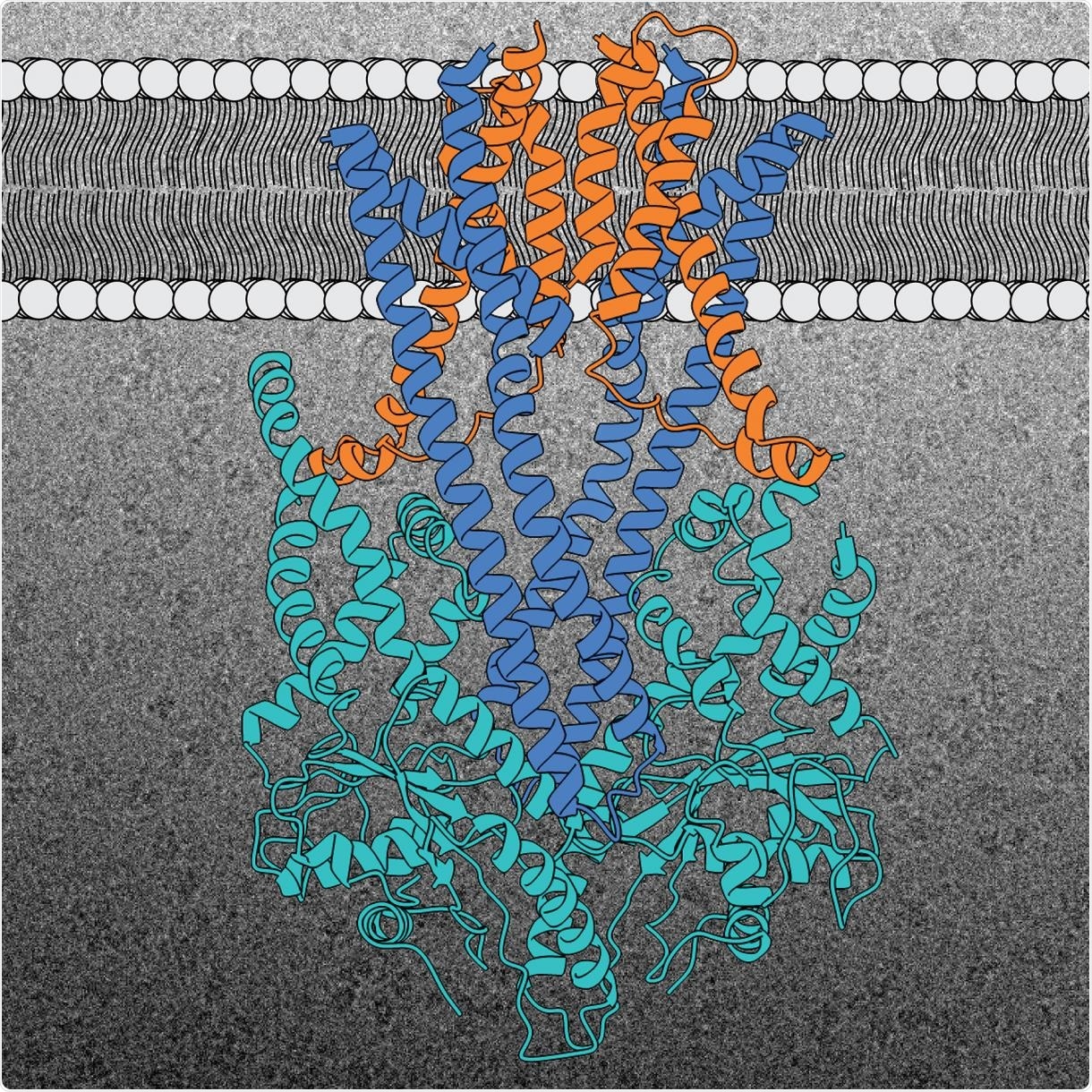
Structure of the GET insertion machine (Get1 in blue, Get2 in orange, and Get3 in light blue). A representative cryo-EM image of the complex is shown in the background. Image Credit: © McDowell and Sinning (2020).
To perform such functions, membrane proteins should be consistently transported from their place of production in the cell to their target and should also be properly embedded into the target membrane.
Scientists from the Heidelberg University Biochemistry Center (BZH) have effectively established the 3D structure of a molecular machine that accounts for the accurate placement of a crucial membrane protein family—the supposed “tail-anchored” membrane proteins, also known as TA proteins.
In an adult human, there are around 100 billion cells, with each one containing an unlimited number of proteins—the players and architects in life that execute a variety of functions. Membrane proteins form a significant part of the proteins in a cell, that is, components of the fine membranes (derived from the Latin word “membrane”) that enclose each cell and also its small organs called the organelles.
Membrane proteins are capable of forming pores or channels and carry out fundamental activities, like signal transmission and transport of substances. Hence, the accurate insertion of a membrane protein is very important for it to perform its biological role and, consequently, for the proper cellular function.
But what are the mechanisms to make sure that the protein truly reaches the right membrane and is incorporated at the right location?
Certain signal sequences—minute portions of proteins that behave similarly to “postcodes”—are crucial for delivery to the right site and for accurate insertion into the membrane. These signal sequences are identified by molecular sorting machines that transport the proteins to their destination.
But in certain proteins, the sequence of signals is found toward the end of the molecule, known to investigators as TA membrane or “tail-anchored” proteins. This membrane protein family is very significant and is involved in several cellular processes, such as membrane fusion and programmed cell death, or apoptosis.
The BZH team headed by Professor Dr. Irmgard Sinning has now established the 3D structure of the molecular machine that introduces the “tail-anchored” proteins into the membrane of the endoplasmic reticulum (ER)—a crucial distribution network within the cell that is linked to all other types of organelles.
For their structural studies, the BZH team employed cryo-electron microscopy (cryo-EM), a technique established by the Nobel Prize for Chemistry back in 2017.
This type of high-resolution structural information is essential to understand the final steps of the protein insertion process into the ER membrane.”
Dr Irmgard Sinning, Professor, Heidelberg University Biochemistry Center
Professor Sinning guides a research team at the BZH.
The GET (short for guided entry of tail-anchored membrane proteins) insertion machine accounts for the accurate insertion of “tail-anchored” proteins into the ER membrane. This insertion machine has hardly changed throughout the evolution from yeast to man, and contains three protein building blocks.
Two protein building blocks are situated in the ER membrane, where they create a kind of cavity called Get1 and Get2. The third cavity, called Get3, is situated outside the membrane and helps deliver the TA proteins.
All three components of the GET insertion machine are crucial for the accurate insertion of the TA protein into the membrane of interest. The Get2 cavity takes the protein from the TA protein deliverer and actually “pushes” it toward the cavity in the membrane interior.
The BZH team demonstrated this surprising detail relating to the interaction between the Get2 and Get3 cavities during their investigation of the protein structure. The researchers also demonstrated that two copies of the insertion machine invariably work closely together to render the integration process more efficient.
The GET insertion machine provides the TA proteins with an energetically favourable route into the membrane.”
Dr Irmgard Sinning, Professor, Heidelberg University Biochemistry Center
“Small membrane proteins like those found in the GET insertion machine are a challenge for structural biology, so our research required innovative ideas,” added Dr. Melanie McDowell, a structural biologist.
Thanks to recent technical improvements in the cryo-EM method, structures of increasingly smaller protein complexes can now be detected in ever greater detail. Therefore, Heidelberg University has established a cryo-EM network (HDcryoNet), rendering the structural analysis of small membrane protein complexes, such as the GET insertion machine, more feasible.
According to Professor Sinning and Dr McDowell, their latest data serves as a major missing puzzle piece needed to complete the whole picture of protein insertion into membranes and protein transport in the cell.
Source:
Journal reference:
McDowell, M. A., et al. (2020) Structural Basis of Tail-Anchored Membrane Protein Biogenesis by the GET Insertase Complex. Molecular Cell. doi.org/10.1016/j.molcel.2020.08.012.