Scientists from the University of Utah School of Medicine have discovered a novel therapeutic target to treat type 1 diabetic patients.
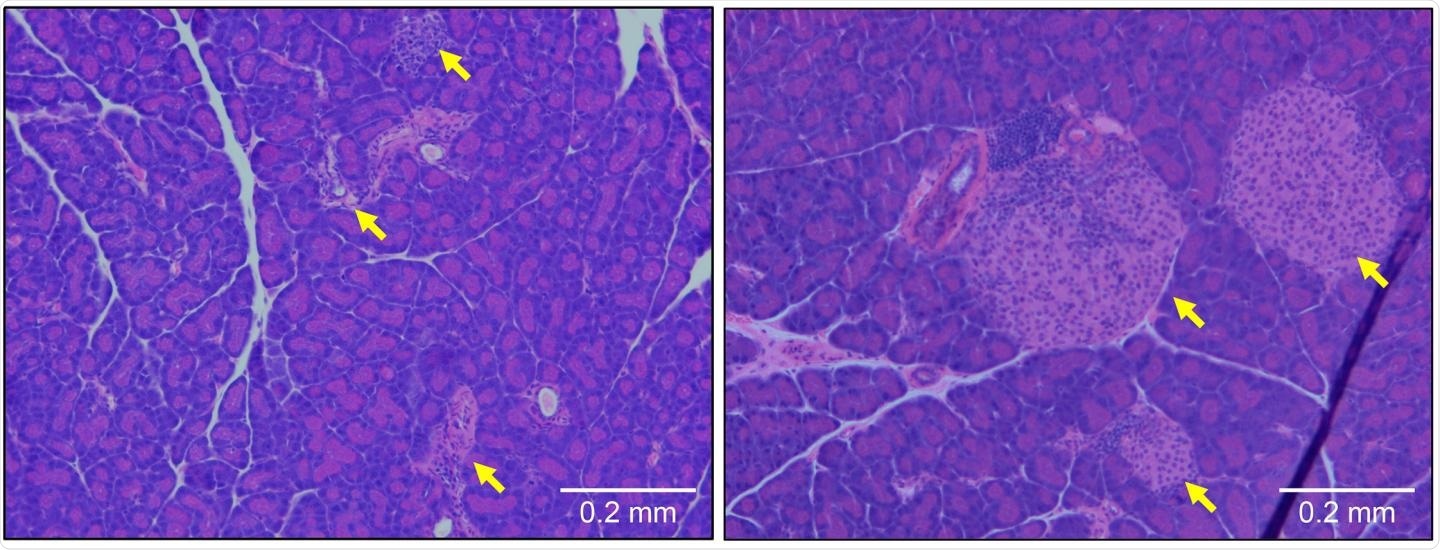
Pancreatic tissue from a type 1 diabetic mouse (left) shows large numbers of infiltrating immune cells and a reduction in the size of β cell-containing islets of Langerhans (yellow arrows). These symptoms are prevented by deletion of the gene encoding OCA-B (right). Image Credit: © 2020 Kim et al. Originally Published in Journal of Experimental Medicine.
The study, which will be published in the Journal of Experimental Medicine on December 9th, 2020, shows that blocking a protein, known as OCA-B, can prevent the development of type 1 diabetes in mice by restricting the activity of immune cells, which otherwise would kill the insulin-producing β cells of the pancreas.
Type 1 diabetes is essentially an autoimmune disease, in which the immune system of the human body mistakenly targets the pancreatic β cells, cutting off insulin production. Patients need insulin therapy throughout their lifetime to maintain adequate blood glucose levels. Currently, there are no therapies that can inhibit the immune system from attacking the β cells, while sustaining its potential to combat infections.
T cells are white blood cells that can detect particular molecules created by invading viruses and bacteria. When these cells come across the specific molecules, called antigens, they switch on a countless number of genes that enable them to combat the infection. OCA-B is a protein that attaches to many of these genes and makes sure these genes are easily reactivated when T cells again encounter the same kinds of antigens at a later time.
In several autoimmune disorders, T cells mistakenly detect and react to antigens generated by normal, healthy cells.
Repeated antigen exposure is a common property of autoimmune responses. We therefore hypothesized that targeting OCA-B would inhibit autoreactive, diabetogenic T cell responses.”
Dean Tantin, Professor, Department of Pathology, University of Utah School of Medicine
Tantin is also a member of the Huntsman Cancer Institute.
In this study, Tantin and collaborators observed that mice that are likely to acquire type 1 diabetes were protected from the disorder if they lacked the OCA-B gene. Potentially autoreactive cytotoxic T cells—which are capable of directly attacking and destroying the pancreatic β cells—continued to remain inactive and did not build up in the pancreas.
Potentially autoreactive helper T cells—which are also capable of recruiting other immune cells to cause a harmful inflammatory reaction—accumulated in the pancreas but continued to remain in a non-responsive condition.
The OCA-B gene controls the function of T cell genes by recruiting the Jmjd1a enzyme to chemically alter the surrounding chromosomal DNA. Along with collaborators, Tantin developed a small peptide that can block the association of the OCA-B gene with the Jmjd1a enzyme and inhibit the reactivation of isolated T cells in the laboratory.
When this peptide was injected into prediabetic mice, it decreased the autoreactive T cells behavior, reduced the inflammation of the pancreas, and restored the blood glucose levels to normal.
While the peptide is unlikely to be used in a clinical setting, it offers a proof-of-principle for OCA-B as a therapeutic target for type 1 diabetes, and can be used as a tool for the further development of therapeutics.”
Dean Tantin, Professor, Department of Pathology, University of Utah School of Medicine
Source:
Journal reference:
Kim, H., et al. (2020) Targeting transcriptional coregulator OCA-B/Pou2af1 blocks activated autoreactive T cells in the pancreas and type 1 diabetes. Journal of Experimental Medicine. doi.org/10.1084/jem.20200533.