AZoLifeSciences talks to Dr. Mo Ebrahimkhani about his research using genetic engineering together with a machine-learning algorithm to mature a lab-grown 'designer liver organoid'.
What led you to begin this research?
During my postdoctoral studies at MIT, I worked on several projects relevant to genetic engineering synthetic biology, and tissue engineering. I also co-led the development of multicellular fetal liver tissue from human induced pluripotent cells.
In my lab at Pittsburgh Liver Research Center, we aim to integrate several areas such as synthetic biology, genetic engineering, and computational analysis to tackle challenges in tissue engineering.
Liver. Image Credit: Magic mine/Shutterstock.com
Please tell us about the machine-learning algorithm that was developed and how it was used to develop your ‘designer liver organoids’?
We did not develop this algorithm which was named CellNet. This program was developed in Patrick Cahan’s lab at JHU and when I started to shape up our studies, I reached out to him to collaborate and he kindly accepted.
The program analyzed synthetic fetal liver tissue and can classify the quality of tissue developed, helping us to understand what genetic elements are missing for the next steps of engineering and maturation.
How were genetic engineering techniques used in your research?
After we identify the genetic circuits via computational analysis, we built the circuits and delivered them to the tissue to program the cells towards the target identity which was the adult liver.
In some cases, we also take advantage of the CRISPR system in collaboration with Samira Kiani’s lab at Pittsburgh Liver Research Center. We used CRISPR to turn on a gene important for liver drug metabolism.
What were you able to show in your mice models?
We showed that when we implanted these engineered designer tissues we could detect human proteins in mouse blood. The function of the liver also helped to improve the survival of the mouse with liver disease.
What stages of development did your lab-grown livers go through and what was this modeled off?
We use human induced pluripotent stem cells and engineer them with genetic circuits at different stages. We can then program them to multiple cells such as hepatocytes and vasculatures. These cells are self-assembled in the form of tissue.
The tissue is going through different steps of liver development similar to native pathways of liver development. It is not identical but captures many facets of development. Part of our approach was inspired by past scientific knowledge about how a liver develops in mice and to some extent humans.
Image Credit: Mesa Studios/Shutterstock.com
Why is your method superior to attempts to mature organs using Petri dishes and growth factors? What are the issues with this technique and how does your method overcome them?
All of the available methods are valuable including treatment with growth factors and they have shown promising results. However, I would say using genetic engineering and synthetic biology can deliver an additional level of control to direct cell fates in the dish.
You can choose which cell to target and how the cells can talk to each other. This is a level of navigation and control that can empower tissue engineering approaches and can be combined with other methods.
How can your algorithms be tweaked to enhance the liver, for example, to protect it from a virus?
It is hard to say. It depends on your goals and objectives.
For example, you can choose to target the genetic materials of a virus if the virus targets the liver tissue using genetic circuits with sensing capacity.
What areas could your designer organoid be used in?
Several applications can be envisioned for designer liver organoids such as drug discovery, human disease models, and also eventually for transplantation as a cell or tissue source.
How and when could this research be used to help people with liver failure?
Both by screening for drugs that can help the liver to regenerate or as a source for tissue and cells that can be produced and transplanted from stem cells. It is hard to estimate an accurate time.
There is more needed to be done -that we are doing now to assess the safety and improve efficacy. It can take several years to address these parts. However, for drug discovery, the tissue can be started to be used right away.
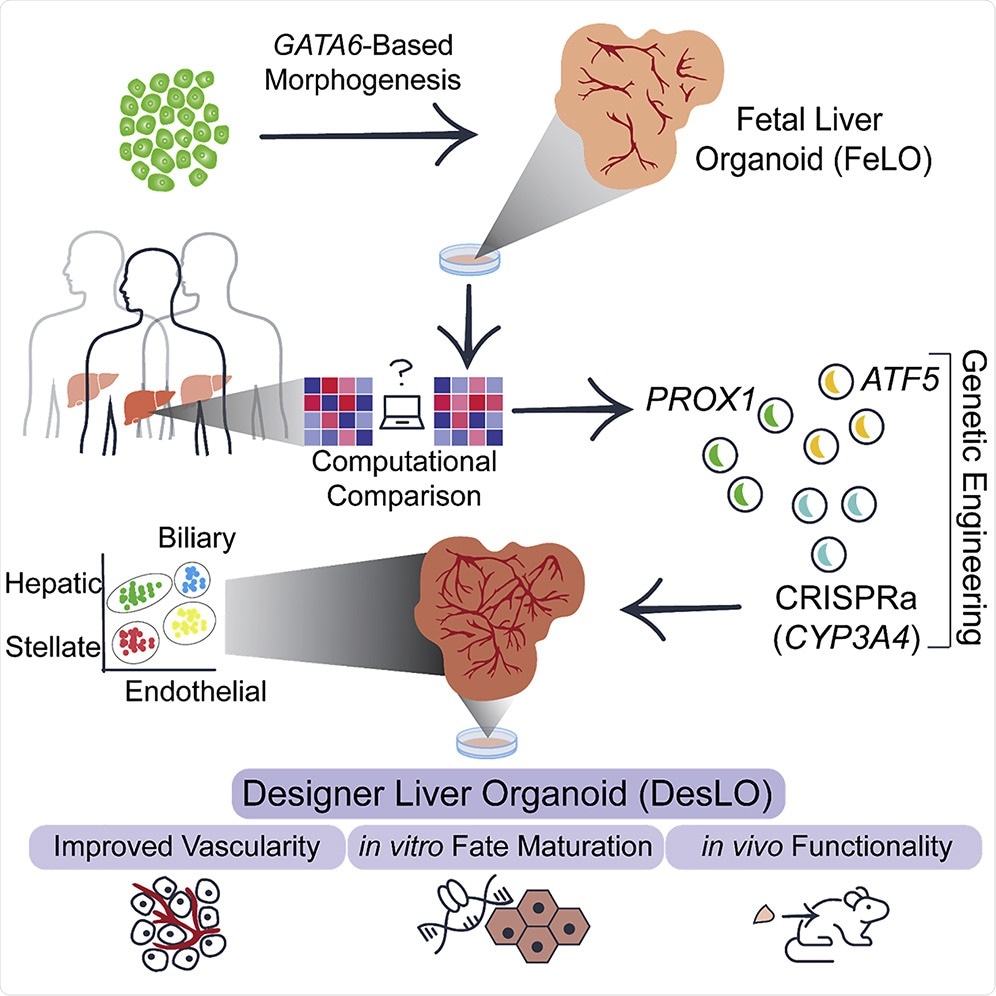
Research. Image Credit: Gene Regulatory Network Analysis and Engineering Directs Development and Vascularization of Multilineage Human Liver Organoids
What other aspects of the human body could this method be applied to?
Obviously, we do not know till we try but there is a good number of evidence that one should be able to combine genetic programming and computational analysis to develop advanced tissues and organoids by genetic designs from stem cells.
What are the next steps for your research?
We will tackle several next steps. Some of these are as follow:
We are collaborating with other scientists to apply the liver organoids for modeling liver disease both genetic disease and acquired ones.
We also think the tissue we have is not perfect there is still the need for more mature liver tissues, and we are working on this area to further mature designer liver organoids towards adult human liver. We also testing the cells in mouse models to improve the therapeutic potentials.
Where can readers find more information?
About Dr. Mo Ebrahimkhani
Mo Ebrahimkhani is an Associate Professor at Pittsburgh Liver Research Center as well as the Department of Pathology, University of Pittsburgh. He is a member of The McGowan Institute for Regenerative Medicine. Before his current position, he was an assistant professor in the School of Biological and Health Systems Engineering at Arizona State University and adjunct faculty of medicine at Mayo Clinic.
He performed his postdoctoral training at the Department of Biological Engineering at Massachusetts Institute of Technology (MIT) and has an M.D. degree from Tehran University of Medical Sciences. He was also awarded a European Association for Study of Liver Sheila Sherlock fellowship to investigate regenerative processes at University College London. His lab combines human stem cells, synthetic biology, and in vivo mouse models to understand tissue development and regeneration and develop technologies to modulate these processes in a personalized fashion.
Mo is the recipient of several research awards from NIH, Mayo Clinic accelerated regenerative medicine award, and New Investigator Award from Arizona Biomedical Research Council.