According to a new study performed by scientists from the University of North Carolina, stimulating an immune signaling pathway, which is best known for combating bacterial and viral infections, can increase the potential of genetically engineered T cells to remove breast cancer in mice.
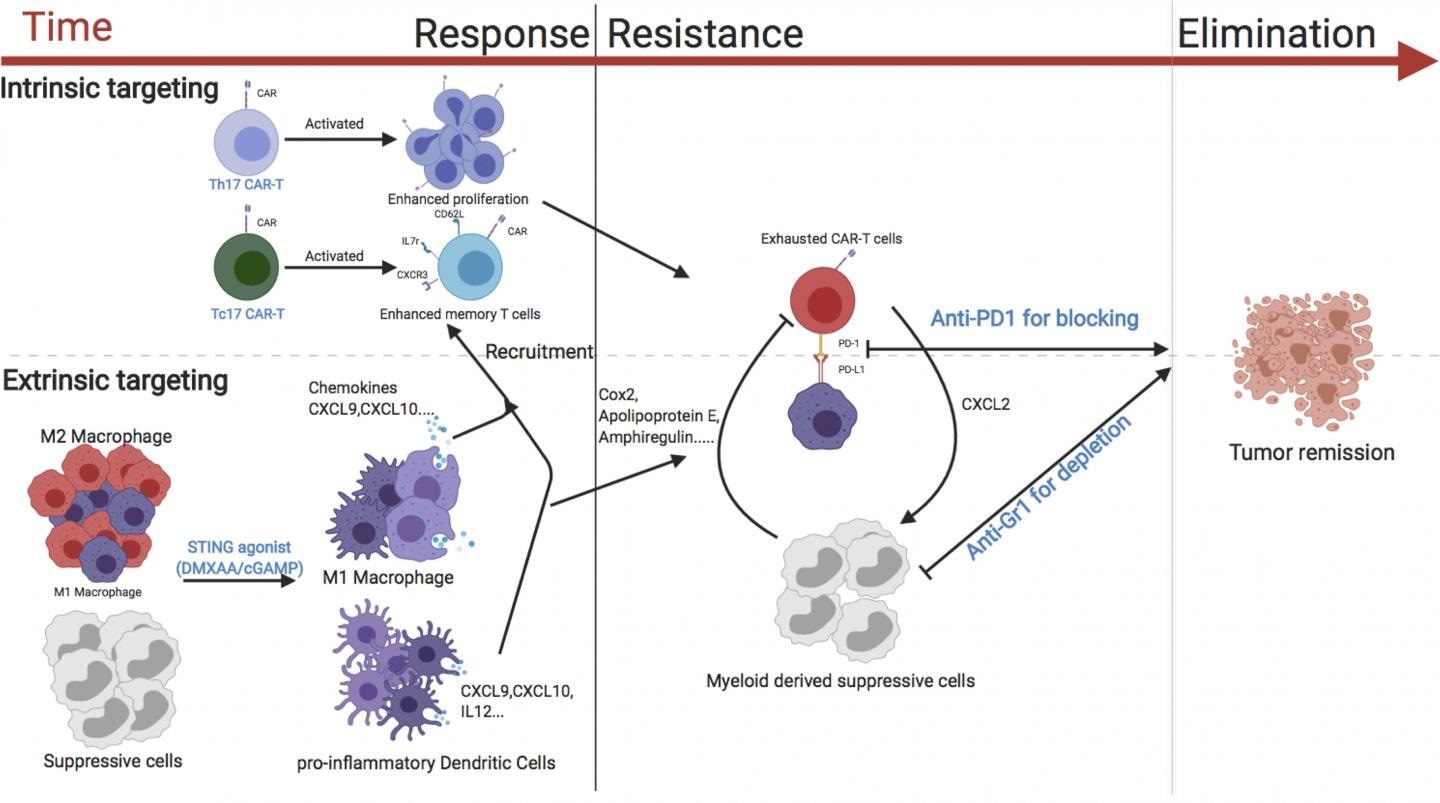
A diagram showing the various strategies that could enhance the activity of CAR T cells against breast cancer. Image Credit: © 2020 Xu et al. Originally published in Journal of Experimental Medicine.
The study indicates that CAR T cells, which are already used for treating specific blood cancers in human beings, may also be effective against solid tumors if paired with other immunotherapeutic methods. The study will be published in the Journal of Experimental Medicine (JEM) on December 31st, 2020.
Chimeric antigen receptor, or CAR, T cells are a form of white blood cells that have been genetically modified to identify and destroy cancer cells that express certain proteins on their surface. These cells have been effectively applied to treat patients afflicted with B cell lymphomas and are presently undergoing clinical trials for the treatment of several other forms of blood cancer.
However, the clinical activity of CAR T cells in patients or animal models with solid tumors has been modest.”
Jonathan S. Serody, Elizabeth Thomas Professor of Medicine, Microbiology, and Immunology, University of North Carolina School of Medicine
Serody is also the Director of the Cellular Therapy Program at the University of North Carolina School of Medicine.
CAR T cells may be not highly effective against solid tumors because they have to travel to the tumors and then should live long enough to destroy all the tumor cells. In addition, the molecules and cells that surround tumors are usually immunosuppressive, stimulating an immune checkpoint that impacts the activity of the CAR T cells.
In the latest study, Serody and collaborators evaluated many approaches to increase the effectiveness of CAR T cells in a breast cancer mouse model. One effective approach was to concurrently treat the mice with medications, like cGAMP, that stimulate the STING pathway—an immune cell signaling pathway that usually causes inflammation in reaction to invading bacteria or viruses.
When the STING pathway was activated, a pro-inflammatory environment was created inside the mouse tumors, and this enhanced the ability of the CAR T cells to accumulate and attack the cancer cells.
This accumulation was specifically great when the mice were injected with CAR T cells that create the immune signaling molecule IL-17A; in comparison to CAR T cells produced using conventional methods.
Serody and collaborators established that the attack made by CAR T cells could be preserved for longer periods provided the mice were also treated with therapeutic antibodies that reduce immunosuppressive cells from the tumor environment and suppress the immune checkpoint from deactivating the CAR T cells. The team discovered that the combination of all of these strategies resulted in the complete removal of breast tumors.
cGAMP is in clinical trials for the treatment of patients with cancer, there are multiple ongoing clinical trials using approaches to inhibit immunosuppressive cells for patients with malignant disease, and there are clinical trials currently evaluating the combination of CAR T cells with immune checkpoint blockade. Together therefore, our data suggest a viable strategy for boosting CAR T activity in solid tumors.”
Jonathan S. Serody, Elizabeth Thomas Professor of Medicine, Microbiology, and Immunology, University of North Carolina School of Medicine
Source:
Journal reference:
Xu, N., et al. (2020) STING agonist promotes CAR T cell trafficking and persistence in breast cancer. Journal of Experimental Medicine. doi.org/10.1084/jem.20200844.