In an effort to regenerate portions of the skull, researchers have successfully used stem cells, corrected the shape of the skull, and thus reversed learning and memory deficits in young mice afflicted with craniosynostosis.
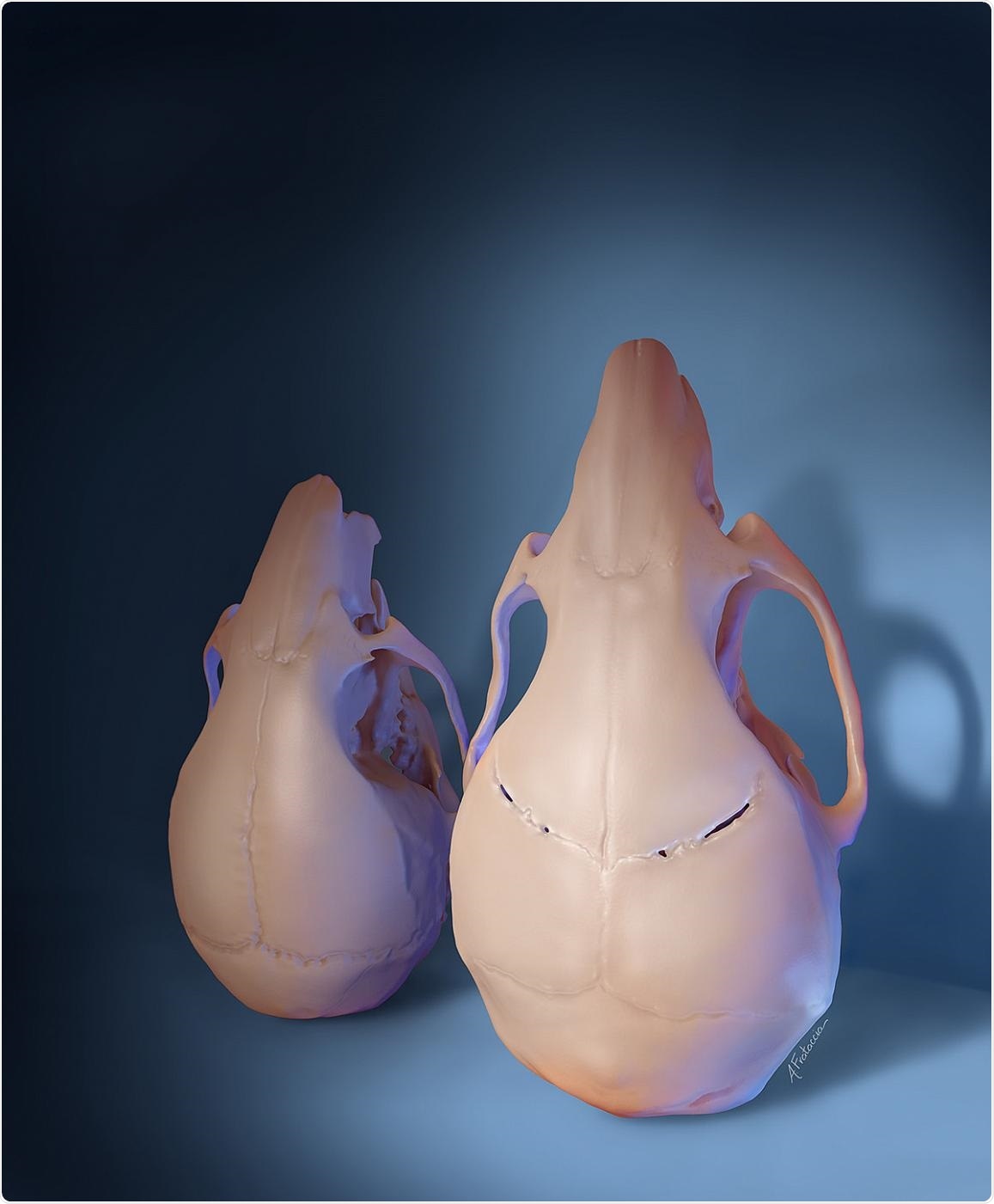
A stem cell-based treatment given to young mice with craniosynostosis regenerated the flexible joints between skull bones and restored skull shape and size (right), compared to untreated animals (left), as shown in this 3D rendering. Image Credit: Amanda Frataccia, University of Southern California.
According to the Centers for Disease Control and Prevention, craniosynostosis is a condition that is estimated to affect one in every 2,500 infants born in the U.S.
The only existing treatment for craniosynostosis involves complex surgery within the first year of life; however, skull defects usually return later.
Supported by the National Institute of Dental and Craniofacial Research (NIDCR), the study could open the way for less invasive and more effective treatments for children suffering from craniosynostosis.
The study results were recently published in the Cell journal on January 7th, 2021. NIDCR is part of the National Institutes of Health.
This is a pivotal study demonstrating both structural regeneration and functional restoration in an animal model of craniosynostosis. It holds great potential for translation to treatment of the human condition.”
Lillian Shum, PhD, Director, Division of Extramural Research, National Institute of Dental and Craniofacial Research
Healthy babies are born with sutures—that is, flexible tissue that occupies the gap between the skull bones—that enable the skull to extend as the brain develops quickly during the first few years of life.
One or more sutures transform into a bone too early in craniosynostosis, closing the space between skull plates and resulting in abnormal growth. The ensuing rise in pressure within the skull may induce physical modifications in the brain, leading to learning and thinking problems.
The connection between changes in the skull and the development of cognitive deficits had not been fully explored. We wanted to know if restoring sutures could improve neurocognitive function in mice with mutations in a gene that causes craniosynostosis in both mice and humans.”
Yang Chai, DDS, PhD, Study Lead and Director of the Center for Craniofacial Molecular Biology a University of Southern California
Chai is also an associate dean of research at the Herman Ostrow School of Dentistry at the University of Southern California, Los Angeles.
The gene, known as TWIST1, is believed to be crucial for the formation of sutures during development. Mutations of this gene may result in Saethre-Chotzen syndrome in human beings. Saethre-Chotzen syndrome is a genetic condition distinguished by various skeletal abnormalities, including craniosynostosis.
To find out if flexible sutures could be restored in mice afflicted with craniosynostosis caused by Twist1 mutations, the team targeted a group of stem cells normally present in healthy sutures. Earlier studies performed by the team suggested that such stem cells—known as Gli1+ cells—are essential to keep the skull sutures intact in young mice.
Moreover, the team discovered that Gli1+ cells are reduced from the sutures of mice that develop craniosynostosis because of Twist1 mutations. Chai and his collaborators reasoned that replenishing the cells may help reproduce the flexible sutures in the affected animals.
To validate this concept, the team added Gli1+ cells collected from healthy mice to a biodegradable gel. The mixture was deposited into grooves intended to regenerate the gap, where skull sutures had existed in mice afflicted with craniosynostosis.
Tissue analysis and skull imaging demonstrated that post six months, new fibrous sutures had developed in the treated areas and that the new tissue stayed intact even after one year. By contrast, the same grooves closed in mice that were given a gel lacking Gli1+ cells.
Upon closer examination, the team found that that Gli1+ cells in the regrown sutures had varied origins—some origins were descended from the implanted cells, whereas others were the animals’ own, after moving from nearby regions. The study results indicate that the implantation of Gli1+ cells party results in suture regeneration by recruiting native Gli1+ stem cells to aid in the process.
Additional experiments demonstrated that untreated craniosynostosis mice had greater pressure within their skulls and displayed poor performance on tests of social and spatial memory as well as motor learning. Following treatment, such measures all returned to levels that were characteristic of healthy mice. The shapes of the skulls of the treated mice were also partly rectified.
Moreover, the treatment reversed the loss of nerve cells and brain volume in regions involved in memory and learning. According to the researchers, these discoveries provide a better understanding of the mechanisms underlying damaged brain function and its enhancement following suture regeneration.
We have discovered that Gli1+ stem-cell-based suture regeneration restores not only skull shape but also neurocognitive functions in a mouse model of craniosynostosis.”
Yang Chai, DDS, PhD, Study Lead and Director of the Center for Craniofacial Molecular Biology, University of Southern California
The team observed that more work needs to be done before this intervention can be tested in human beings, such as studies to establish the optimal timing of surgery and the ideal source and quantity of stem cells.
“This study provides a foundation for efforts to develop a less-invasive, stem cell-based therapeutic strategy that can benefit patients who suffer from this devastating disorder,” Chai concluded.
Source:
Journal reference:
Yu, M., et al. (2021) Cranial Suture Regeneration Mitigates Skull and Neurocognitive Defects in Craniosynostosis. Cell. doi.org/10.1016/j.cell.2020.11.037.