Working in collaboration, Hitachi High-Technologies and Vironova AB (based in Stockholm, Sweden), have begun to explore opportunities where each company’s primary skills and areas of expertise could contribute to the development of new biological drugs (biologics), designed for the life science or pharmaceutical markets.
Hitachi High-Technologies is well regarded for its work within the area of transmission electron microscopy (TEM). New opportunities are arising within this field, due to a growing need for new and improved analytical methods in the pharmaceutical industry.
Vironova is a recognized provider of electron microscopy, working within the pharmaceutical industry, creating proprietary software for the control of TEM systems. Vironova’s primary focus is in the automation of microscope functions, alongside pioneering image analysis via artificial intelligence (AI), machine learning and other new technologies.
In areas like biologics which are stringently regulated, the future of electron microscopy is dependent on objective, automated image analysis. Accurate results coupled with deep, meaningful data will not only improve development time, but this will reduce costs as well.
The combination of Hitachi High-Technologies’ instrument knowhow and Vironova’s automation solutions provide exciting new opportunities in helping to address ever-increasing healthcare needs.
Healthcare Revolution on the Horizon
One of the most challenging issues currently facing humanity is the world’s increasing, and aging population, and the myriad of health problems associated with this (dementia, cancer and the spread of infections from travel and overcrowding). These issues are also exacerbated by the increasing prevalence of multi-antibiotic-resistant bacterial strains.
Meanwhile, a revolution is taking place in pharmaceutical development, thanks to the utilization of biological substances as therapeutics.
Biological drugs make use of a diverse array of substances that have a biological origin. These can include vaccines, recombinant antibodies, gene therapies, and biological molecules like nucleic acids.
The drugs themselves are large molecules - much larger than the small chemicals conventionally used – meaning that they can be examined using an electron microscope. This means that a direct, thorough analysis of numerous characteristics is possible.
The development of biological products is often at the cutting-edge of biomedical research. This may ultimately offer the most successful means of treating medical conditions that currently have no alternative treatment. These products are steadily shifting into the mainstream of drug development, with one specific class of biologics - gene therapies - receiving significant attention for its successes and advances at the moment.
Vironova has considerable expertise in gene therapy applications, with this area being of particular relevance when considering the company’s collaboration with Hitachi High-Technologies.
A viable technical solution has been highlighted by an initial collaborative project, offering a solution which could be attractive to the pharmaceutical industry, while helping to solve analytical bottlenecks in biological drugs development.
Gene Therapy Making a Splash
Following many years of research, the first gene therapy products have been approved by the U.S. Food and Drug Administration (FDA).1 The FDA regulates these products as biologics, granting them twelve-year exclusivity on a non-patent basis.
Two initial products are designed to treat particular types of cancer: Novartis’ Kymriah (a registered trademark of Novartis AG) (tisagenlecleucel) is aimed at children and young adults with a form of acute lymphoblastic leukemia (ALL). This was approved in August 2017.
Meanwhile, Gilead Sciences’ Yescarta (a trademark of KITE Pharma, Inc.) (axicabtagene ciloleucel) is aimed at treating a form of lymphoma. This was approved in October 2017. Both these therapies are types of adoptive immunotherapy with chimeric antigen receptor T cells (CAR-T).
CAR-T cell therapies are combined with immunotherapies and gene therapies. CAR-T uses a patient’s immune cells - called T cells - genetically engineering these to express chimeric antigen receptors (CARs) which are able to recognize cancer cells.
These cells then target and kill cancer cells, following reintroduction into the patient’s bloodstream. There have been some great early successes with CAR-T in clinical trials, and it is hoped that it can be utilized in the treatment of a range of blood and solid tumor cancers.
The Clinical Cancer Advances 2018 report from the American Society of Clinical Oncology’s (ASCO) actually identified CAR-T as the most valuable clinical cancer advance of that year.
Cancer is the primary group of diseases (65%) currently being investigated in gene therapy clinical trials, with the second biggest group (11.1%) being inherited monogenetic diseases. Gene therapy offers the potential to remedy inherited gene disorders by introducing the damaged or missing gene,2 making it unique in this area.
Towards the end of 2017, Spark’s Luxturna (a trademark of Spark Therapeutics, Inc.) was approved to treat children and adults with an inherited eye disease. Here, a single gene therapy treatment in each eye is able to restore the visual cycle by introducing the corrected gene.
Around 2,600 clinical trials and 16 gene therapy products have been approved for use in a variety of countries to date.2,3
In fact, recent clinical successes have resulted in increased investment from the market, prompting an increase in pioneering companies exploring the manufacture and commercialization of new and existing gene therapy products.
Gene therapies are certainly a new and exciting medical paradigm, but these can involve considerable issues in their development and manufacturing, even before they can be used with patients and by medical professionals.
Because of their intended use in patients, gene therapy products should meet strict safety guidelines. As such, there is a clear, pressing need to develop suitable analytical tools for these products.
In 2004, the FDA launched the Process Analytical Technology (PAT) initiative for biopharmaceutical manufacturing. This was designed to emphasize how important it is to ensure detailed analysis during the whole production process, as opposed to the more conventional and limited approach of process validation followed by widespread end-product testing.
Pharmaceutical companies have started to explore new analytical technologies which allow them to measure key quality attributes, thus enabling improved control and understanding throughout the whole manufacturing process.2
Vectors are Critical in Gene Therapy
By introducing genetic material into cells, gene therapy can compensate for abnormal genes, or produce beneficial proteins. Some types of gene therapy utilize a carrier, or vector, which has been specifically genetically engineered to deliver the gene.
Some viruses can also be used as vectors, delivering the new gene by infecting the cell. These viruses are modified so they do not cause diseases.
The vector which is employed to introduce the gene in question is a crucial component of any gene therapy. Generally, viral vectors are used, and these are obtained from adenovirus (AV), lentivirus (LV) or adeno-associated virus (AAV).
A major challenge in the quality control and characterization of viral vectors is their level of complexity. Even the least complex recombinant viral vector - recombinant adeno-associated virus (rAAV) – possesses a more complex structure than the most complex recombinant proteins.
A central factor in developing optimized, large-scale production for viral vectors is ensuring access to reproducible, accurate analytical tools. These must be capable of monitoring quality attributes that guarantee a safe, consistent, effective, high-quality product.
Key quality attributes in the monitoring of viral vector manufacturing for gene therapy include product safety, viral potency, empty viruses, aggregation, purity, quantity, protein content, and identity. Various characterization assays are currently available, with their relevance depending on the virus and the associated expression system.
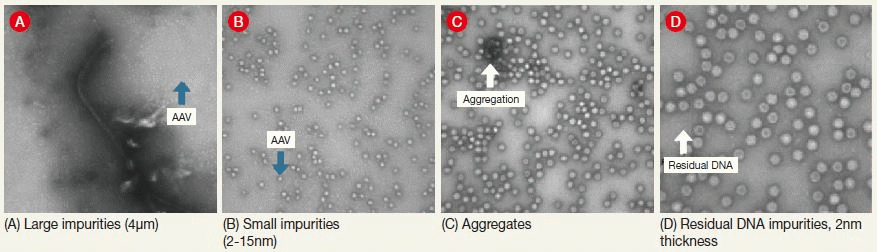
Figure 1. Sample TEM Images. The figure shows examples of the structural expression in transmission electron microscope (TEM) images of contaminants that may appear in, for example, adeno-associated virus samples. Image Credit: Hitachi High-Technologies - Europe
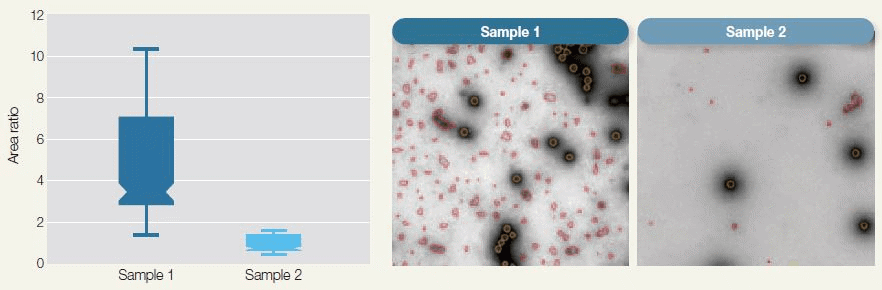
Figure 2. Automated Purity Analysis of Two Samples Using Morphology Classification to Differentiate between Primary Particle and Debris. The box-plot shows purity measurements for the two samples calculated as the ratio of the total area of debris to that of adenovirus particles. Image Credit: Hitachi High-Technologies - Europe
Complexity and Challenge of Monitoring Biologics
Biological substances and structures are often hard to handle. This is because they are sensitive and complex mixtures that are difficult to purify, characterize, or analyze. These mixtures are prone to aggregating, breaking down and becoming contaminated.
Additionally, the route to a working pharmaceutical product is long and complicated, ranging from discovery and the development of reliable processes to effective quality control in regular manufacturing. Many parameters must be appropriately tracked and controlled if undesired issues that could adversely affect the pharmaceutical product are to be avoided.
Characteristics such as purity (see Figure 1 and Figure 2) and integrity (see Figure 3) for example, are frequently and directly correlated to the effectiveness of the final pharmaceutical product. Electron microscopy can contribute significantly in these areas.
The Vital Role of Electron Microscopy in Monitoring Biologics
Electron microscopy imaging is an ideal tool for the investigation of complex biological systems. It offers a combination of metric values and visual proof which cannot be achieved via the indirect methods used for monitoring pharmaceutical processes at present.
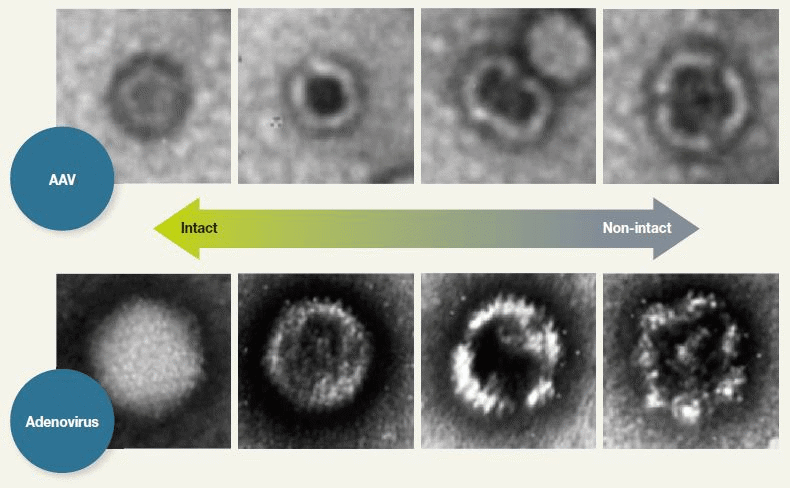
Figure 3. nsTEM for Revealing Particles with Disrupted Capsid Structure. TEM analysis of negatively stained AAV samples (nsTEM) is a reproducible method for revealing particles with disrupted capsid structure. Disrupted particles take in stain and can, therefore, be identified by their darker centers. Image Credit: Hitachi High-Technologies - Europe
It is vital that processes and analyses are automated however if electron microscopy is to be a viable solution suitable for use in the pharmaceutical industry. Automation ensures lower variability, higher reproducibility, and robustness, alongside higher throughput. Quicker access to informative analytical data results in better-informed decisions, as well as earlier process optimization.
Vironova specializes in the automation of microscope operation, alongside innovative image analysis using techniques such as machine learning and AI. This can be viewed as part of a more widespread shift within the pharmaceutical industry towards improved manufacturing efficiency via the implementation of digital technologies, often referred to as ‘Industry 4.0’.4
Initial Collaborative Study Looking at Viruses in Gene Therapy
An initial feasibility study was undertaken using the Hitachi 120 kV TEM system HT7800, working alongside Vironova automation software. In this study, the analysis of viruses in gene therapy was demonstrated via the study of a new type of gene therapy vector named phage therapy.
Phage therapy utilizes bacterial viruses (bacteriophages, or phages) to treat bacterial infections. This practice has been in use for almost a century, but the widespread decline in antibiotic effectiveness has very much renewed interest in this technique.5
The study’s results offered a valuable solution that provided detailed data on sample quality. The learning time required for performing the study was substantially reduced due to the automation of a number of key microscopes operating steps; for example, beam alignment which required just 10% of the usual calibration time.
The sample analysis was considerably less operator-intensive due to automated sample grid screening (illustrated in Figure 4 and Figure 5). The most powerful feature within this solution is the ability to acquire quantitative data via image analysis, meaning that crucial quality attributes of the bacteriophages can be confirmed, with deviations quantified if they exist.
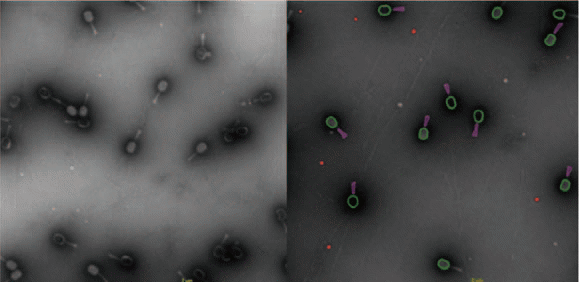
Figure 4. TEM Images of Bacteriophages. The Myoviridae Coliphage samples shown here were isolated by the Brouns lab, Kavku Ubstutyte- BN/TU Delft. Image Credit: Hitachi High-Technologies - Europe
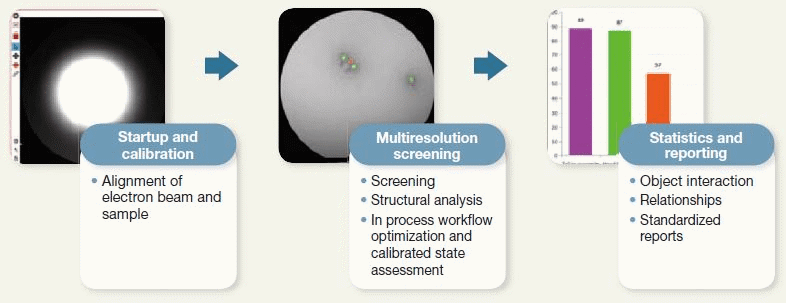
Figure 5. Features of Automation of Microscope Operation and Image Analysis Enabled by the Vironova Software. The automation of some tasks shortened the measurement process. Image Credit: Hitachi High-Technologies - Europe
Opportunity on Front Line of Future Therapeutics
Gene therapy and biologics are emerging disciplines, so production protocols are still being developed and established. Insight is still being gained into the parameters which affect patient safety during gene therapy, simply because the needs of the field are so diverse.
Further challenges arise as more and more projects move into their clinical phases, and as manufacturers develop commercial processes in increasingly large scales.
Ensuring that process steps are optimized to guarantee final product quality and patient safety is a difficult, often daunting task. Access to a wide range of data from reliable analytical solutions is central to the success of this optimization.
TEM can provide images of the sample’s actual content, with automated image analysis subsequently converting the images into accurate metrics that reveal sample quality.
The specific challenges of gene therapy development provide a real opportunity for Hitachi High-Technologies’ existing electron microscopy and Vironova’s automated image analysis platforms to merge into a new offering that can lead this field from the offset while contributing to the potential of this exciting new area.
The work described above is a prime example of the successful integration of cyber technology with the physical world of electron microscopy, with the end goal of helping to address increasing healthcare needs. Everyone will benefit from this collaboration, as it ensures the safest product possible is being manufactured for these innovative therapies.
References and Further Reading
- Nina Forsberg, et al., “Key Considerations in Gene Therapy Manufacturing for Commercialization,” https://cellculturedish.com/GeneTherapyBook/
- Kramberger, et al., “Downstream Processing and Chromatography Based Analytical Methods for Production of Vaccines, Gene Therapy Vectors, and Bacteriophages,” Hum. Vaccin. Immunother, Vol. 11, No. 4, pp. 1010–1021 (2015).
- U.S. Food and Drug Administration, “Approved Cellular and Gene Therapy Products,”
- James Strachan, “Towards Industry 4.0,” (Sep. 2018)
- D. M. Lin,et al., “Phage Therapy: An Alternative to Antibiotics in the Age of Multi-drug Resistance,” World Journal of Gastrointestinal Pharmacology and Therapeutics, Vol. 8, pp. 162–173 (Aug. 2017)
Acknowledgments
Produced from materials originally authored by Martin Ryner, Jonathan Royce and Rhiannon Sanders from Vironova; and Mats Eriksson, Toshihide Agemura, Hiromi Mise and Takashi Kubo from Hitachi High-Technologies.

This information has been sourced, reviewed and adapted from materials provided by Hitachi High-Technologies - Europe.
For more information on this source, please visit Hitachi High-Technologies - Europe.