One of the fundamental principles of biology is the fact that cells are motile and make contact with one another. At the time of embryonic development, cells should interact with their neighbors to identify their right place in the differentiating organism.
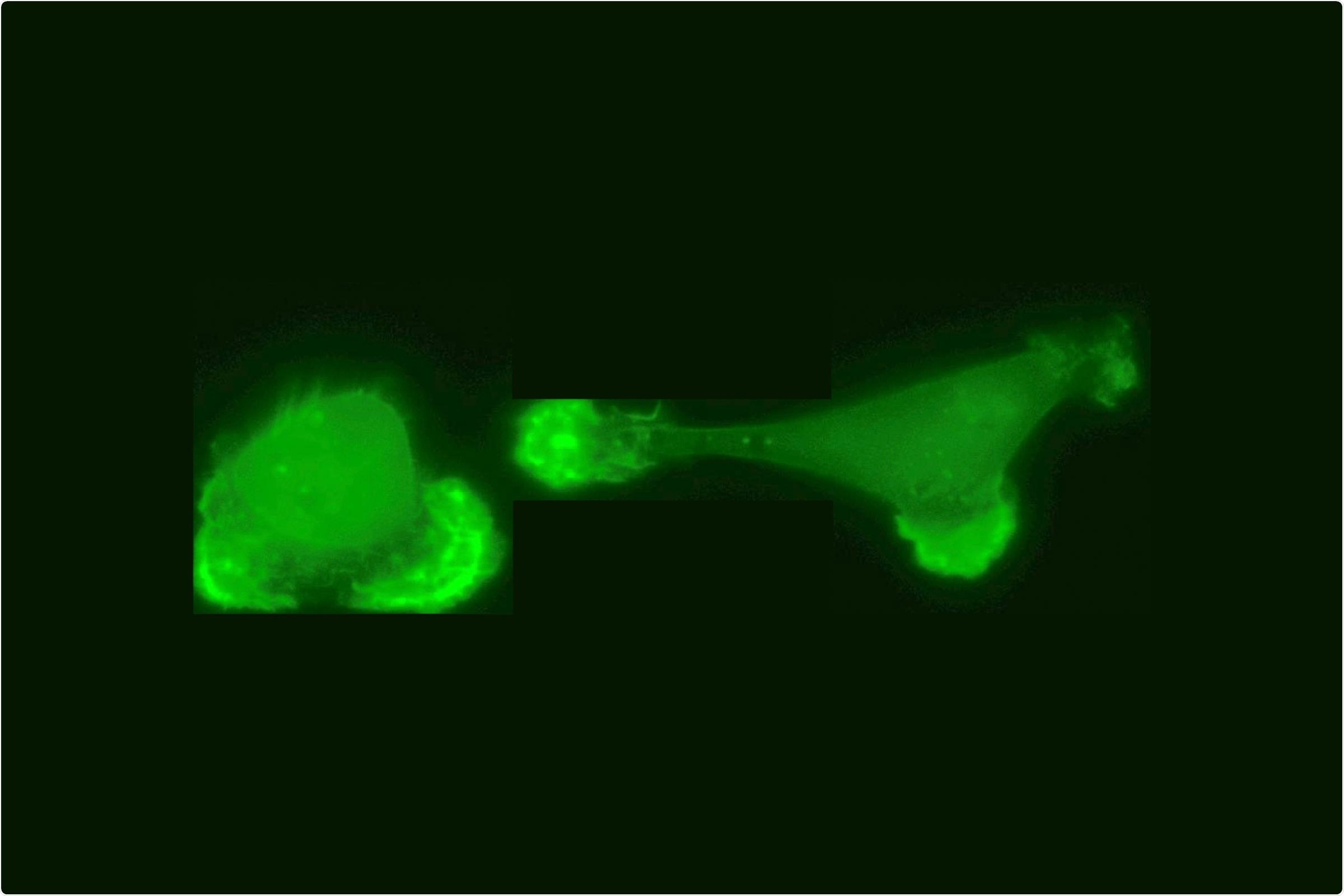
Two interacting breast cancer cells in a cell collider, the actin cytoskeleton of the cells in fluorescent green. Image Credit: © Alexandra Fink, Rädler Group.
Another process is wound healing, wherein direct intercellular interactions are very important. As such, motility allows cells to move to the lesion site and reproduce lost structures. In addition, cancer cells use this trait to exit their site of origin in the primary tumor, which enables them to start the formation of metastatic tumors in other kinds of tissues.
In recent years, biologists and physicists who study motility have mainly focused on investigating how large collectives comprising hundreds or thousands of cells coordinate their movements. We wanted to know how pairs of cells interact when they come into contact, and set out to analyze their behavior using the methods of statistical physics.”
David Brückner, Doctoral Student, Ludwig-Maximilians-Universitaet
Brückner’s PhD supervisor, Chase Broedersz, is an Associate Professor of Theoretical Biophysics at Ludwig-Maximilians-Universitaet (LMU) and the Vrije Universiteit (VU) in Amsterdam.
To do so, Brückner and his collaborators created a microscopic “cage.” which they dubbed a “cell collider.” In cell motility studies, the use of defined geometries is a well-known approach, but Alexandra Fink, a Ph.D. student in the team headed by Joachim Rädler, Professor of Biophysics and the Physics of Soft Matter at LMU, has currently used this strategy in a new context.
According to Brückner “The idea was to isolate two cells while permitting them to interact in a restricted fashion.”
This was accomplished by positioning single cells in each of two compartments linked by a narrow channel. This kind of geometry means that the cells could communicate by expanding thin membrane projections, known as protrusions, and thus unavoidably result in collisions. The nuclei of the cells were labeled fluorescently to track the movements of both cells by fluorescence microscopy so that the positions of the two cells could be monitored over time.
Based on the resulting experimental data, we were able to develop a model that provides a physical description of how the cells interact.”
David Brückner, Doctoral Student, Ludwig-Maximilians-Universitaet
Evasive action on a microscopic scale
The discovery showed that when normal cells make a contact in the cell collider, their protrusions repel one another, and are then completely retracted.
When normal cells make contact, they tend to change direction so as to avoid the obstacle,”
David Brückner, Doctoral Student, Ludwig-Maximilians-Universitaet
This reaction to the initial contact allows the cells to maintain their distance from each other.
Brückner added, “We were surprised to find that tumor cells behave in a very different way.”
In nearly all the cases, the two tumor cells attempted to get past one another. Using their theoretical model, the researchers were able to replicate this reaction in more detail. The study showed that, when a pair of cancer cells approach one another, they do not—as one may anticipate—slow down. But rather, they speed up to get past one another.
Brückner added, “These findings suggest two interesting approaches for further investigations.”
In the following phase, the LMU research team intends to determine the molecular bases for the highly different interactions of the two cell types. It is known that cancer cells vary from their normal counterparts in relation to the groups of proteins exposed on their surfaces. Among these proteins, cadherins—a particular class of proteins that play a crucial role in the mediation of cell adhesion—are of specific interest.
It is still unclear whether or not cadherins alone are responsible for the variations found in this analysis. Additionally, the authors have planned to analyze whether larger cell aggregates, like those detected in tumors, show motility patterns much like those demonstrated by pairs of cells.
Source:
Journal reference:
Brückner, D. B., et al. (2021) Learning the dynamics of cell–cell interactions in confined cell migration. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2016602118.