A research team, headed by scientists from The Scripps Research Institute has made a novel finding that indicates that experimental antibody treatments for Alzheimer’s and Parkinson’s diseases have an accidental adverse impact—brain inflammation—that may need to be addressed if these therapies are to work as planned.
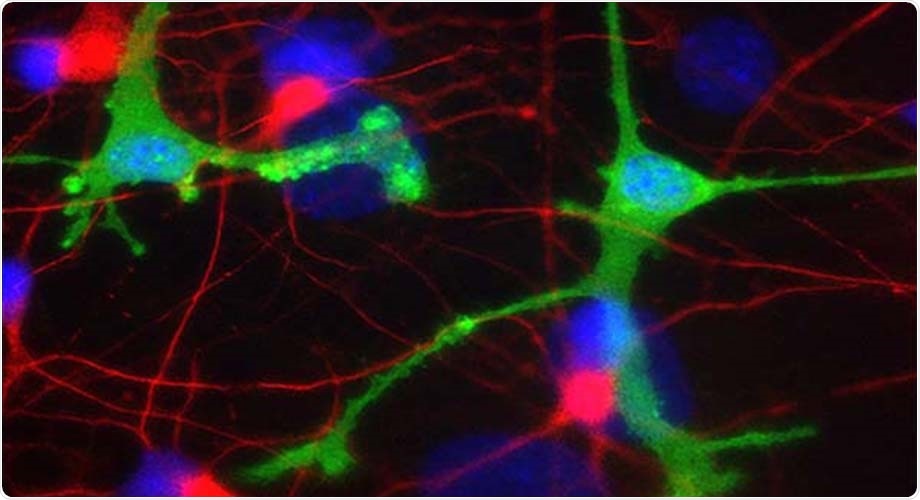
In a new study, antibody drugs in development for two common neurodegenerative diseases triggered inflammation in human-derived brain cells. Microglia cells seen here in green; dopaminergic neurons in red. Image Credit: Lipton laboratory at The Scripps Research Institute.
Experimental antibody therapies for Parkinson’s disease focus on atypical clumps of the protein alpha-synuclein, whereas experimental antibody therapies for Alzheimer’s disease focus on atypical clumps of amyloid-beta protein.
In spite of the promising outcomes in mice, such potential therapies have not yielded much success in clinical trials, to date.
Our findings provide a possible explanation for why antibody treatments have not yet succeeded against neurodegenerative diseases.”
Stuart Lipton, MD, PhD, Study Co-Senior Author, the Scripps Research Institute
Lipton is also the Step Family Foundation Endowed Chair in the Department of Molecular Medicine and the founding co-director of the Neurodegeneration New Medicines Center at The Scripps Research Institute.
According to Lipton, who is also a clinical neurologist, the study is the first to investigate the antibody-induced brain inflammation in a human context. While previous studies were performed in mouse brains, the new study utilized human brain cells.
The study will be published in the Proceedings of the National Academy of Sciences journal of the United States of America during the week of March 29th, 2021.
An approach that may need tweaking
Parkinson’s and Alzheimer’s are neurodegenerative diseases that affect over 6 million Americans. Such disorders are typically characterized by the spread of abnormal clusters of proteins in the brain region, with different combinations of proteins that predominate in different disorders.
An evident treatment approach, which was pursued by pharmaceutical firms in the 1990s, is to administer patients with antibodies that particularly target and remove these abnormal protein clusters, also known as aggregates.
These aggregates include the large clusters that are seen in patients’ brains by pathologists at the time of autopsy and also include clusters that are relatively smaller and harder to detect known as oligomers that are now extensively regarded as the most harmful to the brain.
Precisely how these abnormal protein clusters impair brain cells is a topic of active analysis; however, inflammation is probably a contributing factor. For instance, in Alzheimer’s disease, amyloid-beta oligomers are known to transfer the brain immune cells, referred to as microglia, to an inflammatory state where they can impair or destroy the nearby healthy neurons.
Surprise finding
Lipton and collaborators encountered a surprise finding when they were examining the ability of alpha-synuclein oligomers to activate this inflammatory state: Although the oligomers on their own activated inflammation in microglia extracted from human stem cells, this inflammation was made worse, and not better when therapeutic antibodies were added.
The researchers tracked this effect not to the antibodies as such but to the complexes developed with antibodies and their alpha-synuclein targets.
Amyloid-beta aggregates often exist along with the aggregates of alpha-synuclein observed in Parkinson’s brains, just as alpha-synuclein generally co-exists with amyloid-beta in Alzheimer’s brains.
In the latest research work, the team added amyloid-beta oligomers to their mix, simulating what would occur in a clinical case, and observed that it made the inflammation worse. And adding anti-amyloid beta antibodies made it even worse. The team found that both amyloid-beta antibodies and alpha-synuclein antibodies made the inflammation worse when they effectively struck their oligomer targets.
Lipton pointed out that almost all previous studies of the impacts of experimental antibody treatments were performed with mouse microglia, while the major experiments in this research work were performed with human-derived microglia—in cell cultures or transplanted into the mice brains of mice, whose immune system had been designed to accommodate the human microglia.
We see this inflammation in human microglia, but not in mouse microglia, and thus this massive inflammatory effect may have been overlooked in the past.”
Stuart Lipton, MD, PhD, Study Co-Senior Author, Scripps Research Institute
This kind of microglial inflammation seen in the study could possibly reverse any benefit of antibody therapy in a patient without being clinically evident, added Lipton.
According to him, he and his collaborators have now designed an experimental drug that could be used to offset this inflammation and thus recover any benefit of antibody therapy in the human brain. The team is now actively working on this.