Type I Diabetes Mellitus, also called T1D, is an autoimmune disorder that results in an irreversible loss of insulin-producing beta-cells found in the pancreas.
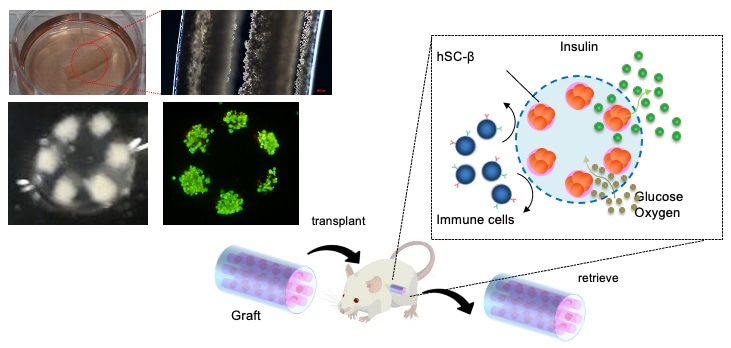
Researchers from The University of Tokyo develop a novel device for the safe and effective transplantation of human-induced pluripotent stem cell (iPSC)-derived pancreatic beta-cells in type I diabetes mellitus. Image Credit: Institute of Industrial Science, the University of Tokyo.
Now, in a recent study, scientists from The University of Tokyo have created an innovative device for the long-term transplantation of iPSC-derived human pancreatic beta-cells.
T1D occurs when autoimmune antibodies kill pancreatic beta-cells, which are essential for insulin production. Insulin controls blood glucose levels, and when it is absent, elevated blood glucose levels gradually impair the peripheral nerves, eyes, and kidneys.
Since the body’s capacity to manufacture insulin decreases over time, the current standard of treatment for T1D is insulin injections. In the last 10 years, an exciting scientific effort has been to explore ways to substitute the lost beta-cells by cell therapy.
Cell therapy is an exciting, but challenging, approach to treat type I diabetes mellitus. The challenge arises from the difficulty to make large amounts of human beta-cells in a dish, and more importantly, to achieve safe and effective transplantation. In this study, we wanted to develop a novel construct that enables successful transplantation of beta-cells in the long-term.”
Shoji Takeuchi, Study Lead Author and Professor, The University of Tokyo
To accomplish their task, the researchers created a lotus-root-shaped cell-encapsulated construct (LENCON) and packed it with human iPSC-derived pancreatic beta-cells, which are an unlimited cell source and help produce any number of beta-cells.
The need for such an encapsulation method comes from the fact that the recipient’s immune cells could damage the newly transplanted cells. To avoid this situation, the researchers built the LENCON graft with millimeter thickness. Previous research works have shown that the millimeter-thick graft diameters reduce the body’s immune reaction to a foreign body.
But the core of cells may not receive nutrients and oxygen at this millimeter thickness; therefore, by utilizing a lotus root shape, the cells were positioned just close to the edge of the graft where nutrients and oxygen could diffuse adequately, providing an atmosphere in which the cells could live, even in the millimeter-thick graft.
After developing the LENCON graft, the question was whether it would effectively regulate blood glucose levels in the long run without triggering an immune response. To answer this question, the team transplanted the construct in diabetic mice with immunodeficiency and immunocompetency.
The former approach made it possible to study the effectiveness of the graft in regulating blood glucose levels even in the absence of an immune reaction, whereas the latter approach addressed both goals.
The investigators discovered that the LENCON graft could sustain normal blood glucose levels in the former mice for over 180 days and could be eliminated without adhesion after more than 12 months of transplantation in the latter mice.
These are striking results that show how LENCON can successfully and safely be used in the setting of type I diabetes mellitus. Our results suggest that LENCON could offer a novel option for cell therapy for type I diabetes mellitus.”
Dr Fumisato Ozawa, Study First Author, The University of Tokyo
Source:
Journal reference:
Ozawa, F., et al. (2021) Lotus-root-shaped cell-encapsulated construct as a retrieval graft for long-term transplantation of human iPSC-derived β-cells. iScience. doi.org/10.1016/j.isci.2021.102309.