A cross-institutional study has led to a new method for the repartition of carbon resources in microalgae from carbohydrates to lipids. It is anticipated that this process will be used to produce biofuel.
Electron microscope image of lipid production in the microalgae Chlamydomonas sp. Image Credit: Kobe University.
This research was possible by the joint work between a research team at Kobe University’s Engineering Biology Research Center, led by Project Assistant Professor Yuichi Kato and Professor Tomohisa Hasunuma et al., and Senior Researcher Katsuya Satoh et al. from the Takasaki Advanced Radiation Research Institute of the Quantum Beam Science Research Directorate (National institutes for Quantum and Radiological Science and Technology).
The study findings were presented in the international academic journal Communications Biology on April 9th, 2021.
Main points
- Microalgae can produce lipids through atmospheric CO2 fixation by photosynthesis, which makes them potential candidates for biofuel production.
- The majority of carbon resources of the microalgae derived from CO2 are collected as carbohydrates (starch) in light/dark environments (i.e. day and night). This makes getting microalgae to manufacture lipids challenging.
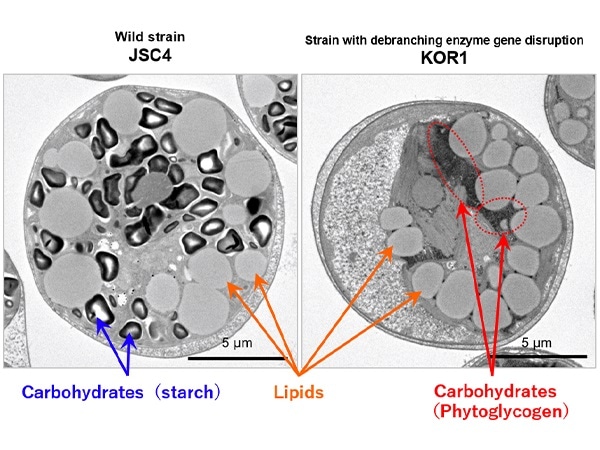
- The researchers used ion beam mutagenesis to create a microalgae strain that can yield significant quantities of lipids even under light/dark environments.
- The starch debranching enzyme gene was inhibited in this microalgae mutant, allowing it to develop phytoglycogen, which is quickly broken down. Carbon resources were then repartitioned from carbohydrate to lipid production.
Research background
Biofuels are renewable resources that have gained a lot of attention in the strive to build more sustainable economies. Microalgae are photosynthetic species that can produce lipids from atmospheric carbon dioxide, which makes them attractive candidates for biofuel development.
However, a Kobe University study team led by Project Assistant Professor Kato Yuichi and Professor Hasunuma Tomohisa et al. found that under light/dark conditions (i.e. day and night), the maximum carbon resources were redirected to starch production rather than lipid production. This is a challenge when growing microalgae outdoors.
Research methodology
Project Assistant Professor Kato and Professor Hasunuma’s research group at Kobe University worked with Senior Researcher Satoh et al. at the National Institutes of Quantum and Radiological Science and Technology (QST) on this research.
To cause mutation in the microalgae, the researchers used an ion beam at QST’s Takasaki Advanced Radiation Research Institute. This allowed them to grow a new mutant strain of Chlamydomonas sp. KOR1, which can create significant amounts of lipids even in light/dark conditions.
The researchers found that this KOR1 strain has defects in the starch debranching enzyme gene ISA1, allowing it to develop a different carbohydrate: phytoglycogen rather than starch.
Microalgae normally synthesize and accumulate carbohydrates (starch) during the day and break them down at night. Many carbohydrates, however, accumulate and cannot be totally broken down.
During the dark time, however, the carbohydrate synthesized by KOR1 (phytoglycogen) was totally broken down. The KOR1 metabolome study showed an overall rise in intermediate metabolites in both the lipid synthesis and starch pathways (intermediate metabolites included glucose-6-phosphate, fructose-6-phosphate, glycerol 3-phosphate, and acetyl-CoA).
The findings shed light on the metabolic mechanism underlying the increased lipid production caused by ISA1 gene disruption. The carbohydrate (phytoglycogen) was easily broken down in the KOR1 strain, and intermediate metabolites caused the carbon resource to be repartitioned to lipid production.
Further developments
It is essential to plant microalgae outdoors in the sunlight to produce biofuels from them. However, under these light/dark circumstances, there is an inevitable decrease in lipid production.
One solution to this issue is the method proposed by this research of “repartitioning carbon resources by destroying the starch debranching enzyme gene.” It is anticipated that this new approach would lead to the large-scale introduction of microalgae-based biofuel production.
Source:
Journal reference:
Kato, Y., et al. (2021) Enhancing carbohydrate repartitioning into lipid and carotenoid by disruption of microalgae starch debranching enzyme. Communications Biology. doi.org/10.1038/s42003-021-01976-8.