T-cells are known to play a crucial role in the human immune system. Through their so-called T-cell receptors (TCR), T-cells detect cancer cells and other harmful invaders in the human body and subsequently activate an immune reaction. However, this recognition process is yet to be fully understood at the molecular level.
The T cell (yellow) touches the antigen-presenting cell. Tiny forces are applied on the surface, eventually the connection breaks. Image Credit: TU WIEN/MEDUNI WIEN.
An interdisciplinary Viennese team of biophysicists, biochemists, and immunologists have now made some fascinating observations.
In a collaborative project financially supported by the Vienna Science and Technology Fund and the FWF, the team investigated the kinds of mechanical processes that occur on detecting an antigen: when T cells travel, their TCRs apply a small force—around 5 pico-newtons (0.0000000005 or 5 x 10−12 newtons)—to pull the antigen.
This force is enough to break down the bonds between the antigen and the TCRs and it also allows the T cells to determine whether they are actually interacting with the antigen they are searching for. The study results have been recently published in the scientific journal Nature Communications.
Tailor-made for a specific antigen
Each T cell recognizes one specific antigen particularly well. To do so, it features around 100,000 TCRs of the same kind on its surface.”
Johannes Huppa, Biochemist and Immunology Professor, MedUni Vienna
When viruses invade the human body, the infected cells present different pieces of viral proteins on their surface. These cells are then examined by T cells to detect the presence of these antigens.
This works according to the lock-and-key principle. For each antigen, the body must produce T cells with matching TCRs. Put simply, each T-cell recognizes only one specific antigen to then subsequently trigger an immune response.”
Johannes Huppa, Biochemist and Immunology Professor, MedUni Vienna
That specific antigen, or to be more precise, any fragment of antigenic protein—that is displayed and exactly corresponds with the TCR of the T cell—can create a slight stable bond. But the question that needs to be addressed by the T cell is how stable is the bond between the receptor and the antigen?
Like a finger on the sticky surface
Let’s say we wish to find out whether a surface is sticky—we then test how stable the bond is between the surface and our finger. We touch the surface and pull the finger away until it comes off. That's a good strategy because this pull-away behavior quickly and easily provides us information about the attractive force between the finger and the surface.”
Gerhard Schütz, Professor of Biophysics, TU Wien
Theoretically, T-cells do precisely the same. Such cells are not static, deform constantly, and their cell membrane is also in a continuous motion.
When a TCR attaches to an antigen, the cell applies a gradually increasing pulling force until the bond breaks down ultimately. This process can give information about whether it is the specific antigen that the cell was searching for.
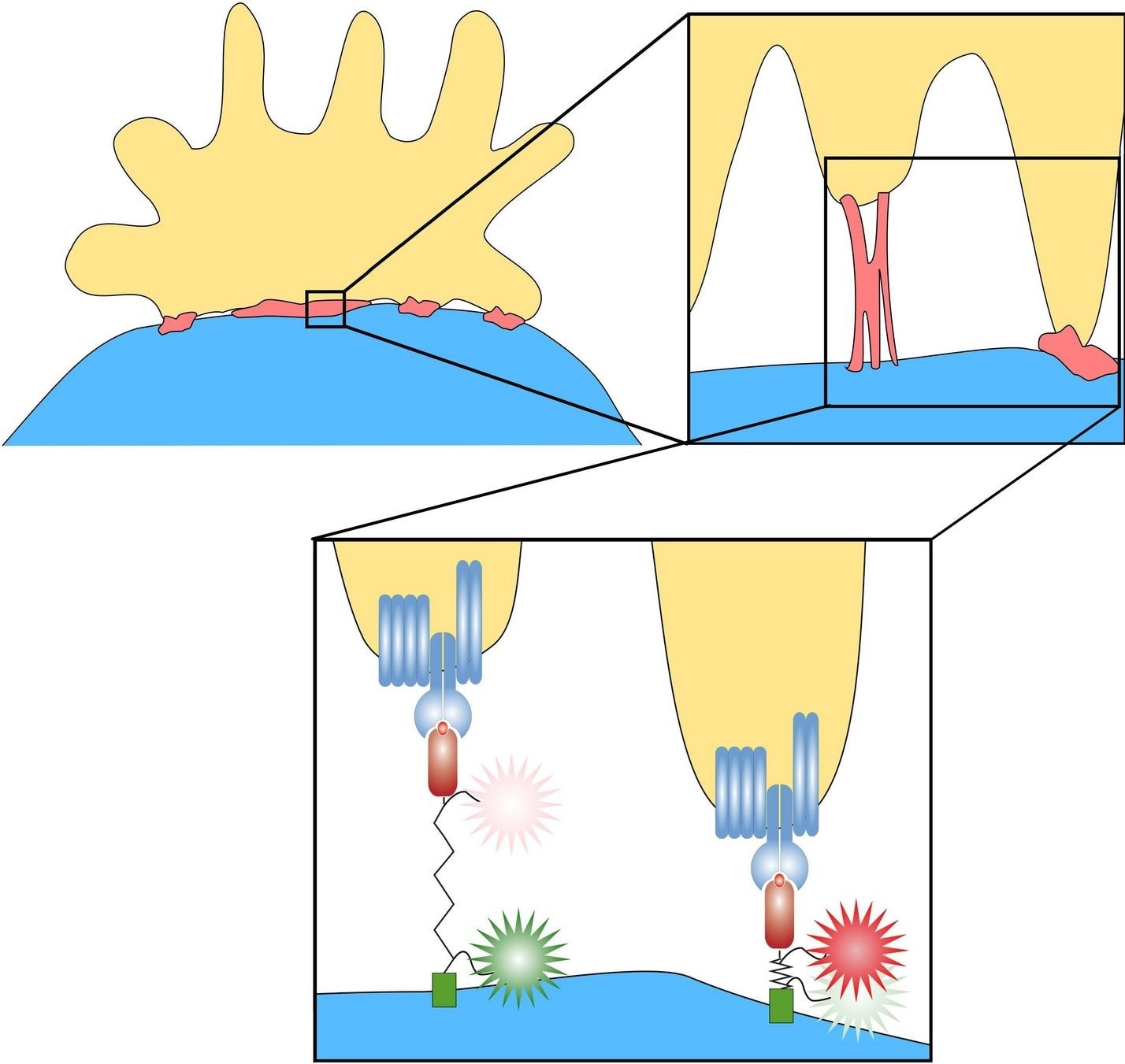
A nano-spring for force measurement
“This process can actually be measured, even at the level of individual molecules,” stated Dr Janett Göhring, who was an active coordinator and the first author of the study from MedUni Vienna and TU Vienna.
“A special protein was used for this, which behaves almost like a perfect nano-spring. The more traction is exerted on the protein, the longer it becomes. With special fluorescent marker molecules, you can measure how much the length of the protein has changed, and that provides information about the forces that occur,” explained Florian Kellner and Dr. Lukas Schrangl, the two other first authors of the study from MedUni Vienna and TU Vienna, respectively.
In this manner, the researchers were able to demonstrate that T cells generally apply a force of up to 5 pico-newtons—a small amount of force that can still detach the receptor from the antigen. By contrast, one would need to pull more than 100 million of these springs at the same time to feel the stickiness with a finger.
Huppa added, “Understanding the behavior of T cells at the molecular level would be a huge leap forward for medicine. We are still leagues away from that goal.”
“But we were able to show that not only chemical but also mechanical effects play a role. They have to be considered together,” concluded Schütz.
Source:
Journal reference:
Göhring, J., et al. (2021) Temporal analysis of T-cell receptor-imposed forces via quantitative single molecule FRET measurements. Nature Communications. doi.org/10.1038/s41467-021-22775-z.