Inside the nuclei of cells, the genome is tightly organized (packaged). This three-dimensional (3D) genome organization is basic because it controls gene expression.
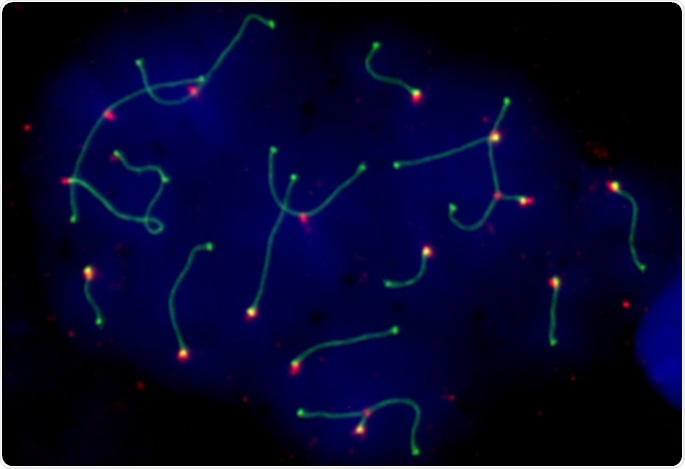
Mouse germ cell (spermatocyte) with chromosomal rearrangements. Image Credit: Covadonga Vara and Aurora Ruiz-Herrera, UAB.
Led by researchers from the Universitat Autònoma de Barcelona (UAB), a new study, using mice models, now shows that the 3D organization of the genome is highly dynamic when male germ cells (precursors of spermatozoa) form and that alterations in this structure can affect fertility.
Published in Nature Communications, the study illustrates the 3D genome organization in germ cells of wild populations of house mice (Mus musculus domesticus) with chromosomal rearrangements, modifications to the genome that alter the structure of chromosomes.
The study denotes considerable progress in the research into mechanisms that produce and regulate the structure and function of the genome when gametes (oocytes and sperm) form.
Organisms that reproduce sexually tend to produce haploid gametes (with one single set of chromosomes) by two successive cell divisions preceded by just one round of genome replication.
When this process (known as meiosis) occurs, the genome organization is tightly controlled to enable recombination—a basic mechanism that maintains the organism’s genetic diversity through the exchange of the homologous chromosomes of the progenitors, while enabling the resulting chromosomes to be integrally transmitted, with no changes in their structure and/or number, to the subsequent generation.
The study shows that both the dynamics of the genome organisation during the formation of gametes and the recombination is affected by the presence of chromosomal rearrangements.”
Aurora Ruiz-Herrera, Research Head and Associated Professor, Department of Cellular Biology, Physiology and Immunology, Institute of Biotechnology and Biomedicinem, UAB
“We detected that these chromosomal rearrangements affect chromosome folding within the nucleus of the germ cells, thus altering the pairing pattern of homologous chromosomes and meiotic recombination. These results will pave the way for new investigations into the genetic mechanisms responsible for infertility,” added Ruiz-Herrera.
The results point to the importance of the three-dimensional genomic context in which the recombination takes place, where factors such as chromosomal reorganizations can shape the genomic makeup of a given species.”
Covadonga Vara, Study Co-Author, UAB
Vara is a member of the research group coordinated by Aurora Ruiz-Herrera.
The researchers add that finding out the mechanisms that control the structure and function of the genome during gametes formation is fundamental. This is because the deregulation of this process can lead to disorders like infertility and a change in the number of chromosomes, for example, trisomy-21.
Source:
Journal reference:
Vara, C., et al. (2021) The impact of chromosomal fusions on 3D genome folding and recombination in the germ line. Nature Communications. doi.org/10.1038/s41467-021-23270-1.