The surfaces of many immune cells contain chemokine receptors that play a critical role in their function. Chemokines are essentially small proteins that adhere to these chemokine receptors and regulate the behavior and movement of white blood cells. But despite the significance of this family of receptors, not much is known about their activation mechanism.
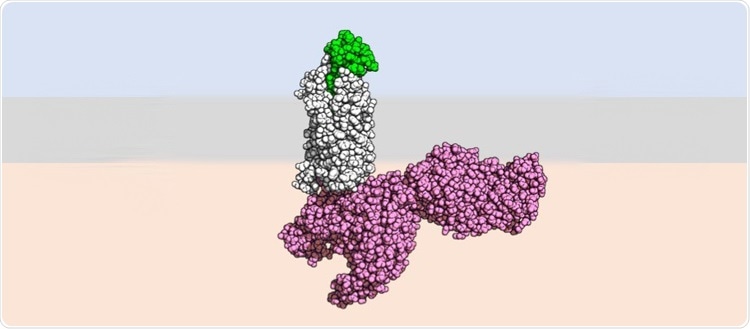
Stylized image of the active form of the receptor (in white) bound to a modified chemokine (in green). In pink, a part of the cell signaling machinery. Image Credit: UNIGE- Laboratoire Hartley.
A research consortium from the University of Geneva (UNIGE), the Biozentrum of the University of Basel, and the Paul Scherrer Institute (PSI) in Villigen, all based in Switzerland, has successfully decoded the activation mechanism of the CCR5 receptor, a member of the family of chemokine receptors implicated in many diseases, such as cancer, HIV/AIDS, and the respiratory complications of COVID-19.
This latest finding helps understand the biology of chemokine receptors and provides valuable insights for improving novel drugs. The study has been published in the Science Advances journal.
The receptor CCR5 plays an important role in immune defense and inflammation. It has been a major target for anti-HIV drugs for a long time. Stephan Grzesiek, a professor at the Biozentrum of the University of Basel, co-directed the study along with Professor Oliver Hartley from the Department of Pathology and Immunology at UNIGE Faculty of Medicine, and collaborators from the PSI.
Research on CCR5 began almost 25 years ago as part of the fight against AIDS. It is indeed fundamental to the HIV infection mechanism, but also seems to be very important in many other pathological processes, notably in cancers and inflammatory diseases. However, in order to better exploit it for therapeutic purposes, we needed to understand, at an atomic level, how activation through its binding to chemokines works.”
Stephan Grzesiek, Professor, Biozentrum, University of Basel
Chemokines, the small signaling molecules. play a vital role in the circulation and stimulation of immune cells. They bind to the receptors located on the membrane of white blood cells, acting as guides. The chemokines direct the cells to the right place at the right time, maintaining the immune system and, at the same time, responding to injury or infection.
But there was no clarity on how the receptor can sense the docking of a chemokine at the outside of the cell, and how this activation message gets transmitted to the inside of the cell so that the cell could respond.
Visualizing atomic structures in 3D
To date, further study of this phenomenon was hampered by the difficulty of visualizing the 3D structures of the receptors when attached to the molecules that stimulate them. As such, the team from the University of Basel that specializes in structural biology used cryo-electron microscopy tools. These tools make it viable to preserve and visualize the structure of even the smallest elements of living organisms.
However, in order to understand the entire process, it is necessary to make use of engineered chemokines that bind to receptors more stably than the natural ones. For this, we were able to exploit the molecules that we had discovered in the course of our HIV drug research.”
Oliver Hartley, Department of Pathology and Immunology, UNIGE Faculty of Medicine
It was observed that some variants indeed over-activate the receptor, while others block them completely.
The right key to fit in the lock
The receptor, integrated into the cell membrane, operates similar to a “lock and key” mechanism. A certain part present in the chemokine receptor should fit into the CCR5 lock to stimulate a change in the receptor structure. This subsequently triggers the activation and migration of white blood cells.
The activation capacity of chemokines is determined by certain amino acids (protein building blocks) that must arrange themselves in a specific pattern. If this part of the chemokine adopts a straight shape, it succeeds in activating the receptor. But if the amino acids are changed, the molecule adopts a slightly different shape which, although it maintains a very strong bond with the receptor, prevents its activation.”
Oliver Hartley, Department of Pathology and Immunology, UNIGE Faculty of Medicine
These slight changes, therefore, make the difference between receptor inhibitors and activators.
Better-targeted and therefore more effective drugs
While the architecture is almost identical, slight structural variations between the engineered chemokines determine their potential to inhibit or activate the receptor. Further understanding of this mechanism would enable drug improvement by developing novel compounds that can fine-tune the immune system.
Source:
Journal reference:
Isaikina, P., et al. (2021) Structural basis of the activation of the CC chemokine receptor 5 by a chemokine agonist. Science Advances. doi.org/10.1126%2Fsciadv.abg8685.