Scientists from Tokyo Metropolitan University have found that a particular chemical feature of a crucial protein, called tau, may cause it to build up in the brain and lead to various disorders, like Alzheimer’s disease.
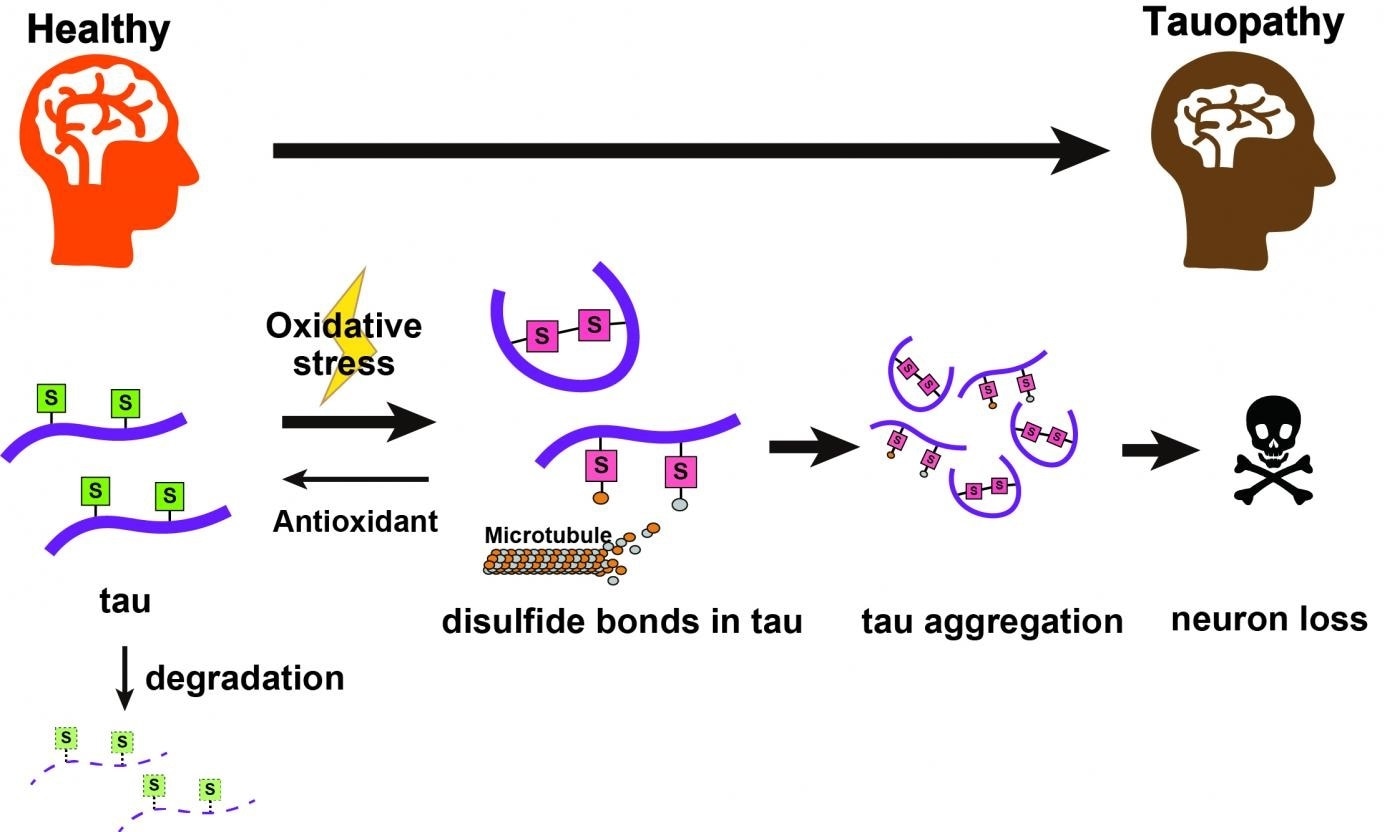
Tau proteins with cysteine groups bearing thiol groups (S) undergo chemical changes under oxidative stress to form disulfide bonds, making a toxic mutant of the tau protein that can aggregate. These go on to cause neural degeneration. Antioxidants can help reduce these back to thiols; these normal tau proteins can then be naturally cleared away by the cell. Image Credit: Tokyo Metropolitan University.
The researchers observed that disulfide bonds on specific amino acids act to stabilize the tau protein, which leads to its accumulation. And this accumulation becomes worse with increased oxidative stress. The detection of chemical targets that trigger the accumulation of the tau protein may lead to innovative treatments.
The tau protein plays a key role in the healthy function of biological cells. It helps in the formation and stabilization of microtubules—the thin filaments that crisscross the interiors of cells to help keep them structurally stiff and offer “highways” to shuttle molecules between organelles. But when the microtubules are not formed correctly, they can build up and form sticky clumps.
These aggregates in the brain block the firing of neurons and lead to many neurodegenerative disorders, called tauopathies. Alzheimer’s is one such disease. This makes it highly crucial to identify the “switch” that converts tau from a vital part of cell function to a causative pathology.
The research team, headed by Associate Professor Kanae Ando from Tokyo Metropolitan University, has been utilizing model organisms, such as the Drosophila fruit fly, to reveal how certain features of the tau protein disrupt its proper activity.
Flies can be genetically modified to express the same tau proteins expressed in human beings. By carefully altering the parts of the tau-encoding gene, the researchers have been attempting to identify how some features of mutant tau proteins influence their behavior.
In the new analysis, the researchers observed that modifications to amino acid residues in the protein called cysteine in two different sites—C291 and C322—had a drastic impact on the toxicity and amount of the tau protein. In an additional breakthrough, the researchers emphasized down the chemical feature that was responsible for rendering them harmful to the normal function of cells, in other words, disulfide bonds formed by the cysteine groups.
The harmful accumulation of the tau protein became worse when cells were kept in an environment with increased concentrations of reactive oxygen species, as thiol groups present on the cysteines were oxidized to create disulfide bonds.
Biochemical conditions with increased oxidative stress resemble those seen in patients with tauopathies. The joint expression of anti-oxidants to offset this effect allowed natural processes to remove tau proteins, leading to significantly lower tau levels.
The researchers expect that this understanding of which chemical groups account for tau toxicity may result in innovative treatments that prevent or reduce tau accumulation, thereby helping people with tauopathies worldwide.
Source:
Journal reference:
Saito, T., et al. (2021) Disulfide bond formation in microtubule-associated tau protein promotes tau accumulation and toxicity in vivo. Human Molecular Genetics. doi.org/10.1093/hmg/ddab162.