Mitosis, the mechanism of cell division, is an age-old process. Yet, the process has not been fully understood or replicated. It is a vital process and involves over 100 proteins at its core.
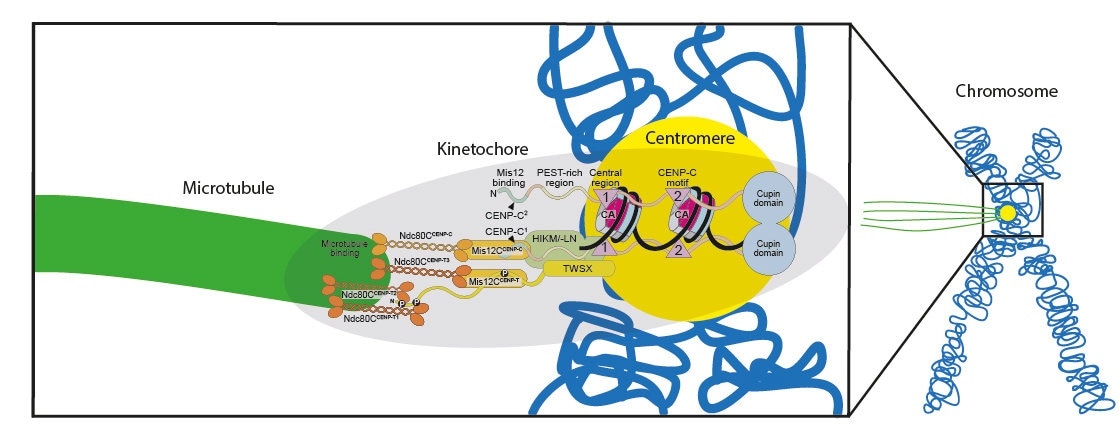
Scheme of the reconstituted kinetochore binding the centromere (yellow) of the chromosome (blue) on one side and a microtubule (green) on the other side. Image Credit: MPI of Molecular Physiology.
A research team from Max Planck Institute of Molecular Physiology in Dortmund, headed by Prof. Dr Andrea Musacchio, fully reconstituted the kinetochore—the engine of the mitosis machinery.
Effective reconstitution of the kinetochore is the first step towards the making of artificial chromosomes, which can be used to restore missing cell functions. The research findings were recently published in the journal Science Advances.
A wonder of nature
During cell division, the 23 chromosomes in human cells duplicate into identical copies that remain joined at a region known as the centromere. The kinetochore, a complex assembly of proteins that binds to thread-like structures called microtubules, is present in the centromere. During the progression of mitosis, the kinetochore signals the microtubules to tear the DNA copies apart, towards the newly forming cells.
The kinetochore is a beautiful, flawless machine: You almost never lose a chromosome in a normal cell! We already know the proteins that constitute it, yet important questions about how the kinetochore works are still open: How does it rebuild itself during chromosome replication? How does it bind to the microtubules? And how does it control them?”
Dr Andrea Musacchio, Professor and Director, Mechanistic Cell Biology, Max Planck Institute of Molecular Physiology
A life’s endeavor
Dr. Musacchio started seeking answers 20 years ago and was guided by a simple motto—“Before we understand how things go wrong, we better understand why and how things work.” Hence, he tried to rebuild the kinetochore in vitro. In 2016, he synthesized a partial kinetochore formed of 21 proteins.
Musacchio, postdoc Kai Walstein, and their colleagues at MPI Dortmund successfully reconstructed the system—all subunits, from the ones that bind the centromere to the ones that bind the microtubules, are now present in the correct numbers and stoichiometry.
The researchers demonstrated the proper functioning of the newly developed system by successfully substituting parts of the original kinetochore in the cell with artificial ones.
This is a real milestone in the reconstruction of an object that exists, unaltered, in all eukaryotic cells since more than one billion years!”
Dr Andrea Musacchio, Professor and Director, Mechanistic Cell Biology, Max Planck Institute of Molecular Physiology
This innovation can open the door towards the making of synthetic chromosomes carrying functions that can be replicated in organisms. “The potential for biotech applications could be huge,” added Musacchio.
In the protein factory
The researchers faced a major hurdle while rebuilding the kinetochore—the complete reconstruction of the highly flexible Centromeric Protein C (CENP-C). This protein is important as it bridges the centromeric region to the outer proteins of the kinetochore. The scientists rebuilt CENP-C by “gluing” together both its ends.
The reconstitution of complex protein assemblies requires a highly organized laboratory, like a factory. For every kinetochore protein, the researchers built a production pipeline for gene isolation, expressed them in the cells of insects, and collected them.
When we put them together in vitro, these proteins click-in to form the kinetochore, just like LEGO pieces following the instructions.”
Dr Andrea Musacchio, Professor and Director, Mechanistic Cell Biology, Max Planck Institute of Molecular Physiology
Every kinetochore protein had a different interface and interaction with closer proteins.
The research team further intends to investigate the function and interaction of kinetochore in the presence of microtubules and supplied energy (in the form of ATP).
Source:
Journal reference:
Walstein, K., et al. (2021) Assembly principles and stoichiometry of a complete human kinetochore module. Science Advances. doi.org/10.1126/sciadv.abg1037.