A new study published in the journal Oncotarget reports the use of single-cell, force spectroscopy methods to probe biophysical and biomechanical kinetics of breast, brain, pancreatic, and prostate cancer cells using standard chemotherapeutic drugs in hypoxia and normoxia over 12–24 hours.
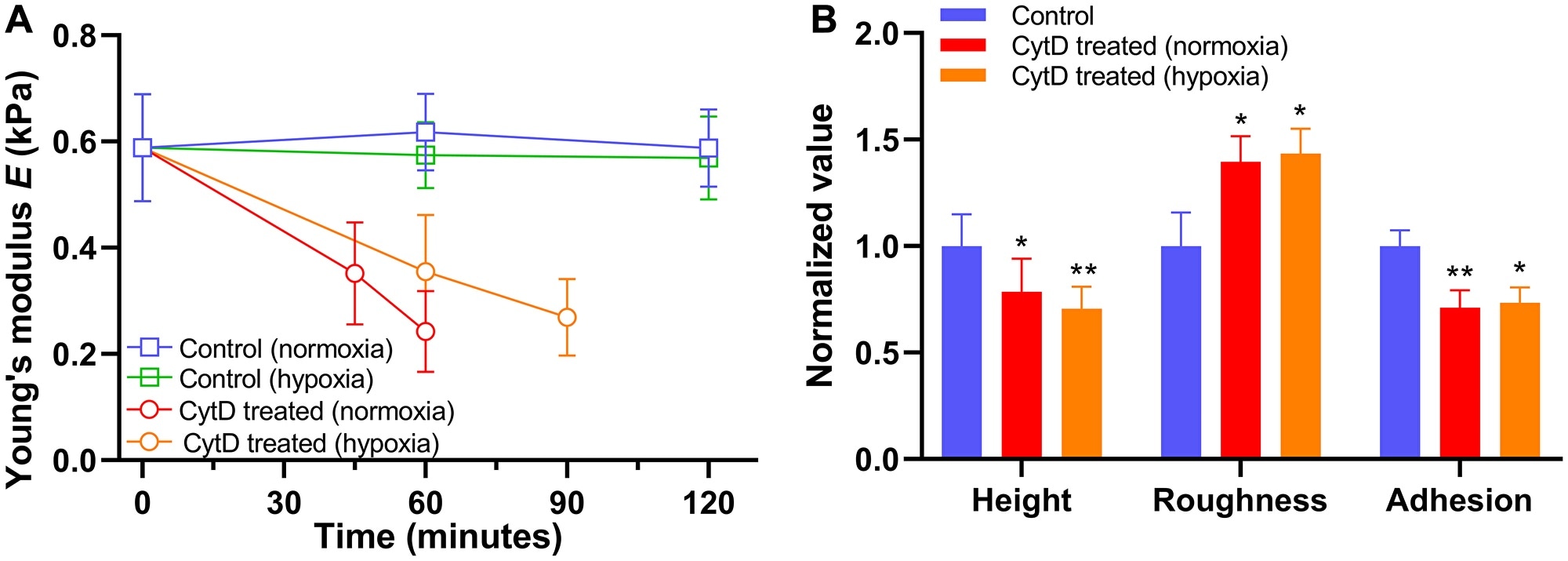
Alteration in biomechanical properties of PANC-1 cells exposed to 5 μM cytochalasin D (CytD) in normoxia and hypoxia. (A) Time trace of Young’s modulus E in normoxia (n = 5) and hypoxia (n = 5) after exposure to cytochalasin D. (B) Normalized values of cellular height, roughness, and adhesion measured after exposure to cytochalasin D in normoxia for 60 minutes (n = 5) and hypoxia for 90 minutes (n = 5). Data are mean ± s.d., Repeated measured one-way ANOVA, post-hoc Tukey test; ns, not significant; *P < 0.05; **P < 0.01; ****P < 0.0001. Statistics between normoxia and hypoxia showed no statistical difference (ns). Image Credit: Oncotarget.
The researchers found that upon exposure to the drugs, breast, brain, and pancreatic cancer cells turned less stiff by about 55%–75%. By contrast, prostate cancer cells turned stiffer. This is because of either reinforcement of cytoskeletal structure or drug-induced disruption.
But the rate of change in the stiffness was found to plunge up to two folds in hypoxia, indicating a correlation between drug resistance of cancer cells and cellular stiffness in the hypoxic tumor microenvironment.
Moreover, the researchers found major changes in the cell surface roughness, cell body height, and cytoadhesion of cancer cells upon exposure to drugs, which followed the stiffness trend.
The findings demonstrate that a degree of chemotherapeutic drug effects on biophysical and biomechanical properties of cancer cells can be differentiated in hypoxia and normoxia, which are correlated with changes to the cytoskeletal structure and integrity during the drug-induced apoptotic process.
Cell surface plays important roles in fundamental cellular functions such as signaling, communication, adhesion, transport, and tumor metastasis.”
Dr Yongki Choi, The North Dakota State University
The cell surfaces tend to interact dynamically with biological, physical, and chemical environments that surround cells. Thus, any changes in the surface structure of the cell considerably affect the overall cellular functions.
Specifically, the deformability of cells related to cell shape, invasion, and motility has demonstrated implications for cancer metastasis and cell death, which is vital information for making new anticancer drugs with higher efficacy in cancer chemotherapy.
Various studies have demonstrated the relationship between chemotherapy-induced cell death and changes in cellular mechanics like stiffness, but the effect of drugs on biophysical and biomechanical properties of cancer cells is not completely understood so far.
Moreover, stiffness at the tissue level is largely influenced by the stage of the tumor, invasiveness, and location within the tumor because of the deposition of the extracellular matrix, which affects the cellular behavior and metastatic capacity also at the single-cell level.
A number of studies based on the AFM-based force measurements have also indicated a major change in cell stiffness with higher metastatic efficiency in human cancer cell lines and chemotherapy exposure in leukemia cells.
In this study, the researchers measured the effects of the drug on the biophysical and biomechanical properties of four cancer cell lines: PANC-1 pancreatic cancer, MDA-MB-231 triple-negative breast cancer, U-118 MG glioblastoma cell lines, and PC-3 prostate cancer.
In the Oncotarget Research Output, the Choi Research Team reported that changes in biomechanical parameters of cancer cells that were exposed to an inhibitor of actin polymerization cytochalasin D in normoxia and hypoxia were examined.
The PANC-1 cells turned less stiff upon extended exposure to cytochalasin D, and the decrease in stiffness was slower in hypoxia than normoxia.
Moreover, morphology and non-specific binding force measurements of the cells exposed to cytochalasin D exhibited a decrease in cell height and cellular adhesion, but an increase in cellular roughness.
Similar alterations in biomechanical properties of other cancer cell lines treated using cytochalasin D and chemotherapeutic drugs indicate that the drug-induced cytotoxicity is partially because of dynamic changes in the cytoskeletal structure.
Generalizing the effects of the drug on the biophysical and biomechanical parameters of cancer cells is challenging, but a combination of these parameters could enable the drug-induced apoptotic process in normoxia and hypoxia to be identified and differentiated.
Source:
Journal reference:
Alhalhooly, L., et al. (2021) Dynamic cellular biomechanics in responses to chemotherapeutic drug in hypoxia probed by atomic force spectroscopy. Oncotarget. doi.org/10.18632/oncotarget.27974.