Researchers at Mayo Clinic have used sophisticated imaging technology to offer unmatched insights into the BRCA1-BARD1 protein complex, often mutated in patients with breast or ovarian cancer.
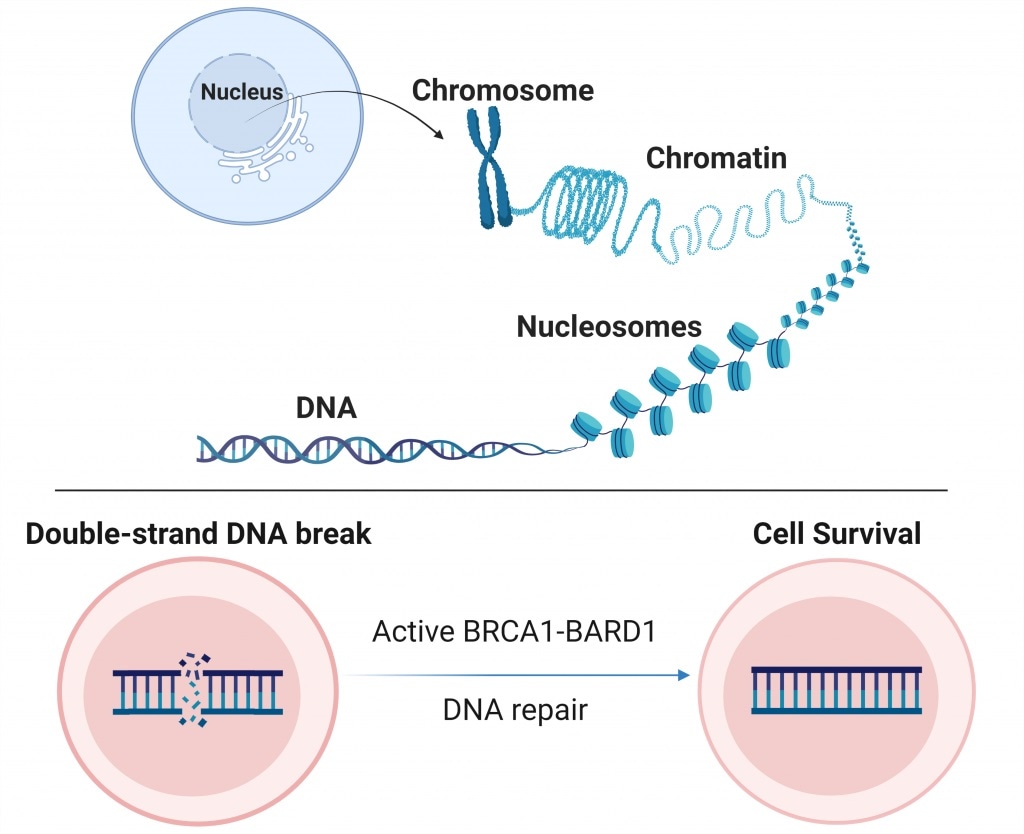
Image Credit: Mayo Clinic, created with BioRender.
Published in the Nature journal, the study identifies aspects of how BRCA1-BARD1 functions, promoting future translational research, drug development, and cancer prevention efforts.
“BRCA1-BARD1 is important for DNA repair. It has direct relevance to cancer because hundreds of mutations in the BRCA1 and BARD1 genes have been identified in cancer patients,” stated Georges Mer, Ph.D., the lead author of the study and a structural biologist and biochemist at Mayo Clinic.
But no one knows if these mutations, or variants of unknown significance, are cancer-predisposing or not because we do not know whether the variants are located in a region of BRCA1-BARD1 that is important for function. Now because we can see how BRCA1-BARD1 works, we have a good idea of what regions of BRCA1-BARD1 are important for function.”
Dr Georges Mer, Structural Biologist and Biochemist, Mayo Clinic
Within a cell, the complex of histone proteins and DNA are tangled into the so-called chromatin and packaged into bundles known as nucleosomes.
Proteins responsible for DNA damage response must access chromatin to repair damaged DNA. BRCA1-BARD1 works to fix broken DNA strands, thus helping to maintain cells and their survival. However, it is also a function that could probably be inactivated or inhibited if this is the approach used by a cancer cell to survive chemotherapy.
Cryo-electron microscopy and nuclear magnetic resonance spectroscopy
We used two techniques—cryo-electron microscopy and nuclear magnetic resonance spectroscopy—to understand at near-atomic resolution how BRCA1-BARD1 associates with the nucleosome, the repeating unit of chromatin, and how BRCA1-BARD1 modifies chromatin.”
Dr Georges Mer, Structural Biologist and Biochemist, Mayo Clinic
Cryo-electron microscopy involves flash-freezing purified BRCA1-BARD1 attached to nucleosomes, together denoted as macromolecules, which are then imaged with the help of an electron microscope. The macromolecules are oriented in different ways inside the sample. Thus, a computer program assesses all the orientation data to develop a 3D structure.
Moreover, Dr. Mer and his colleagues evaluated BRCA1-BARD1 nucleosome complexes using nuclear magnetic resonance spectroscopy, which involves using a powerful magnet to investigate the relative positions of atoms inside macromolecules. The researchers could use these imaging tools to visualize BRCA1-BARD1 in action and unravel a new function of the complex.
We showed how BRCA1-BARD1 attaches ubiquitin to the nucleosome, but we also determined that BRCA1-BARD1 recognizes ubiquitin already attached to the nucleosome, which serves as a signal for broken DNA. We discovered an unexpected cross-talk by which ubiquitin recognition by BRCA1-BARD1 enhances its ubiquitin attachment activity, and this helps us better understand how BRCA1-BARD1 performs its function.”
Dr Georges Mer, Structural Biologist and Biochemist, Mayo Clinic
The team made a video from the cryo-electron microscopy data to indicate where the protein complex interacts with the nucleosome.
From discovery science to patient care
Dr. Mer and his colleagues anticipate that high-resolution images of BRCA1-BARD1 can help promote patient care and future treatment of cancer in two ways: classification of variants of unfamiliar significance and directing more accurate drug development.
“With these 3D structures, we should be able to convert several variants of unknown significance to likely cancer-predisposing variants,” stated Dr. Mer. “This work is also expected to have an impact on drug development in the long term because the 3D structures of BRCA1-BARD1 in complex with the nucleosome we generated may help in the design of small molecules that could, for example, inactivate BRCA1-BARD1.”
Source:
Journal reference:
Gough, R. E., et al. (2021) Talin mechanosensitivity is modulated by a direct interaction with cyclin-dependent kinase-1. Journal of Biological Chemistry. doi.org/10.1016/j.jbc.2021.100837.