Scientists from Singapore have found that the brain lacks the ability to maintain the cholesterol-rich myelin sheath that safeguards and insulates neurons when a protein named TDP-43 is absent.
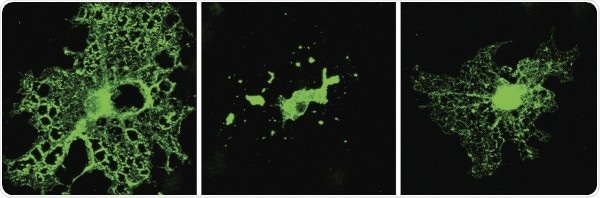
Compared with a normal cell (left), an oligodendrocyte lacking TDP-43 (center) produces less myelin (green) because it is unable to synthesize or take up sufficient amounts of cholesterol. Supplementing TDP-43–deficient cells with cholesterol (right) restores myelin production. Image Credit: ©2021 Ho et al. Originally published in Journal of Cell Biology.
Published on August 4th, 2021, in the Journal of Cell Biology (JCB), the study reports that restoring cholesterol levels could be a new therapeutic strategy to treat diseases related to TDP-43.
The TDP-43 protein is associated with various neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). TDP-43 has several key roles inside cells; however, under certain conditions, it tends to clump together to develop harmful aggregates that damage cells and inhibit TDP-43 from carrying out its normal functions.
The brains of a majority of ALS patients and ~45% of FTD patients feature TDP-43 aggregates. These aggregates are also associated with many other neurodegenerative disorders, including certain cases of Alzheimer’s disease.
Apart from being formed in neurons, the aggregates also form in other types of brain cells like oligodendrocytes. The oligodendrocytes safeguard neurons and accelerate nerve impulse transmission by wrapping neurons in a fatty substance known as myelin.
Shuo-Chien Ling and the team from the Yong Loo Lin School of Medicine, National University of Singapore earlier demonstrated that oligodendrocytes require TDP-43 to survive and wrap neurons in myelin.
Specifically, we found that mice with oligodendrocytes lacking TDP-43 develop progressive neurological phenotypes leading to early lethality. These phenotypes were accompanied by the death of oligodendrocytes and progressive loss of myelin.”
Shuo-Chien Ling, Yong Loo Lin School of Medicine, National University of Singapore
As part of the new study, Ling and team identified that oligodendrocytes turn dysfunctional in the absence of TDP-43 because they cannot synthesize or absorb the cholesterol they require to sustain myelin synthesis.
Cholesterol forms a key component of myelin—that 25% of the body’s total cholesterol is found in the central nervous system. It is well known that oligodendrocytes produce large amounts of cholesterol for themselves. However, they can also obtain it from other brain cells named astrocytes.
Ling and team identified that when TDP-43 is absent, oligodendrocytes lack several of the enzymes needed to produce cholesterol while exhibiting lower levels of the low-density lipoprotein receptor that can absorb cholesterol from outside the cell. When these TDP-43–deficient cells were supplemented with cholesterol, their ability to maintain the myelin sheath was restored.
Patients may manifest similar defects in cholesterol metabolism, where aggregate formation might inhibit TDP-43 from carrying out its normal functions. Ling and team investigated brain samples from FTD patients and identified that their oligodendrocytes synthesized lower amounts of two main enzymes needed for cholesterol synthesis, while the low-density lipoprotein receptor was integrated into TDP-43 aggregates.
Our results indicate that simultaneous disruption of cholesterol synthesis and uptake is likely one of the causes of the demyelination phenotype observed in mice with TDP-43–deficient oligodendrocytes, and suggest that defects in cholesterol metabolism may contribute to ALS and FTD, as well as other neurodegenerative diseases characterized by TDP-43 aggregates.”
Shuo-Chien Ling, Yong Loo Lin School of Medicine, National University of Singapore
Thus, drugs with the ability to modulate cholesterol metabolism might prove to be an innovative therapeutic approach to treat such diseases, the researchers noted.
Source:
Journal reference:
Ho, W. Y., et al. (2021) TDP-43 mediates SREBF2-regulated gene expression required for oligodendrocyte myelination. Journal of Cell Biology. doi.org/10.1083/jcb.201910213.