Researchers from Johns Hopkins Kimmel Cancer Center in their recent research elucidate why certain drugs, in clinical trials, for treating a kind of acute myeloid leukemia (AML) often fail and revealed a means to restore their efficacy.
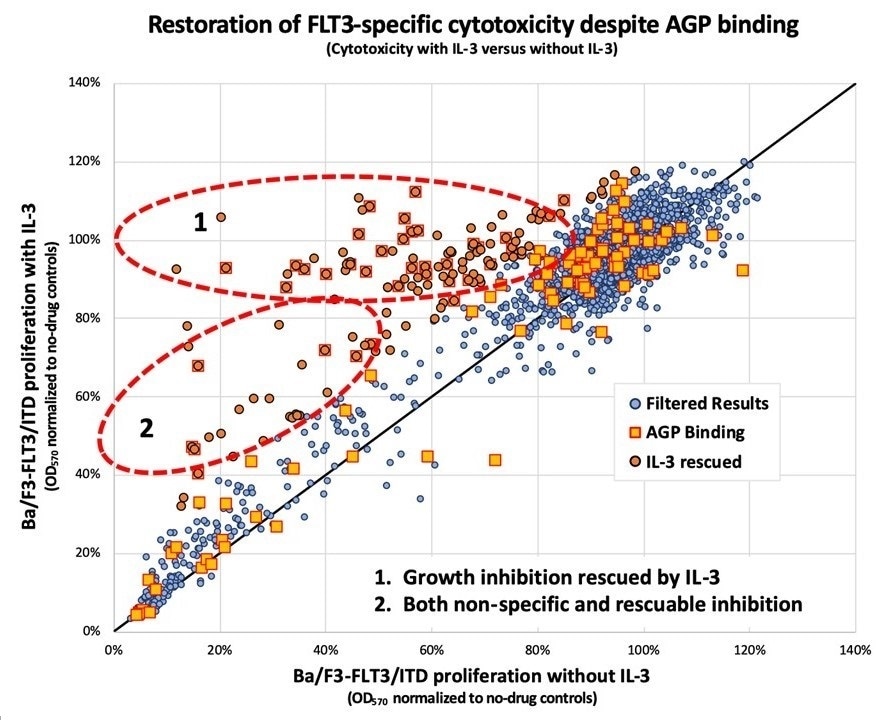
2,800 compounds from the Johns Hopkins Drug Library were screened for the ability to restore the activity of midostaurin in the presence of inhibiting amounts of human AGP. Most compounds had no effect upon FLT3 (blue dots falling along the diagonal line); however, a subset of the compounds (orange dots) appear to restore the ability of midostaurin to inhibit FLT3 and kill the cells. The authors also screened the compounds for their ability to bind to AGP (squares). Compounds that both restore activity and bind to AGP (squared orange dots) may serve as AGP decoys to rescue midostaurin and other similar drugs from AGP inhibition. Image Credit: David Young MD, PhD and Donald Small MD, PhD.
The preclinical study probably removes a pharmacologic barrier in creating molecularly targeted therapies for AML. The study was published recently in the journal Blood Cancer Discovery.
Around one-third of patients with AML possess a mutation in the gene FLT3. Normal FLT3 genes secrete an enzyme that signals bone marrow stem cells to divide and replenish. Upon mutation, the FLT3 triggers fast growth of leukemia cells, resulting in greater rates of relapse after treatment and lessens survival overall.
FLT3-mutated AML is especially sensitive to a class of drugs named e-family tyrosine kinase inhibitors (TKIs), making them major candidates for drug development, elaborates the first author of the study, David Young MD, Ph.D., who researched while at the Johns Hopkins Kimmel Cancer Center. Dr Young is currently at the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Mostly, these TKIs and others fail, resulting in patients’ relapse. Researchers from the Kimmel Cancer Center carried out a series of experiments with human leukemia cell lines and mice and revealed that the human alpha(1)-acid glycoprotein (AGP) attaches the drug, successfully inhibiting it from arriving at its intended FLT3 mutation target and destroying the cancer cells.
Donald Small MD, Ph.D., director of the Division of Pediatric Oncology and the Kyle Haydock Professor of Oncology at the Johns Hopkins Kimmel Cancer Center, and co-workers treated mutant FLT3 cell lines.
The mutant FLT3 cell lines were cultured in human plasma from healthy donors or standard lab conditions with lestaurtinib, TTT-3002, or midostaurin a drug recognized by the Food and Drug Administration (FDA) that targets FLT3—at different concentrations. Plasma, the clear part of the blood, consists of proteins and other non-cellular factors.
The researchers discovered that when human plasma is added, it decreased the capability of TKI to inhibit FLT3, contrary to blood components from other sources. Additional examinations pinpointed human AGP as binding the three drugs and inhibiting their capability to destroy leukemia cells.
To illustrate the clinical relevance of the results, the scientists gathered blood samples from adults recently diagnosed with AML and observed the effect of their plasma on midostaurin. In the presence of high inflammation, as in newly diagnosed leukemia patients, AGP levels are high. As anticipated, the drug lost effectiveness in the human plasma assay from the cases.
Midostaurin is very specific and potent, and we’ve seen about a 10% improvement in patient outcomes since the FDA approved its use in adults with AML in 2017. But we never got the ‘home run’ we were looking for because it’s bound by AGP.”
David Young, Study First Author, National Heart, Lung and Blood Institute, National Institutes of Health
The researchers, in another series of experiments, demonstrated that this plasma protein inhibition can be reversed by introducing an agent that attaches to AGP. Mifepristone is recognized to attach to AGP with an affinity equivalent to or greater than that of the three drugs used in the research.
Scientists ran the FLT3 assay with human protein plasma, mifepristone, and midostaurin. They discovered that mifepristone replaced the AGP-bound midostaurin, reviving its anti-FLT3 activity. Testing the concept in mice, they had similar results.
We wanted to free up enough midostaurin to allow the drug to do its thing. If we give human AGP, and give midostaurin plus mifepristone, it kills the leukemic cells. Mifepristone acts like a decoy that prevents midostaurin from binding to the glycoprotein.”
David Young, Study First Author, National Heart, Lung and Blood Institute, National Institutes of Health
Even though there is a need for further testing and validation, the scientists state that mifepristone or other agents with equivalent AGP-binding properties can be analyzed in future clinical trials of combination TKI therapies, or generated as protein plasma “decoys” to amplify the efficacy of molecularly targeted therapies.
Screening the Johns Hopkins Drug Library—a compilation of around 3,000 FDA-recognized compounds and drugs, retained by study co-author Jun Liu Ph.D., co-director of the cancer chemical and structural biology program at the Johns Hopkins Kimmel Cancer Center—provides tantalizing assurances of more drugs that might act like mifepristone to restore anti-FLT3 activity and might integrate with TKI therapies in numerous ways.
There may be ways to affect the pharmacology of the human body to give these old drugs new life.”
David Young, Study First Author, National Heart, Lung and Blood Institute, National Institutes of Health
Source:
Journal reference:
Young, D. J., et al. (2021) A Method for Overcoming Plasma Protein Inhibition of Tyrosine Kinase Inhibitors. Blood Cancer Discovery.