Hepatitis C virus thrives in humans for years, damaging the liver by causing chronic inflammation, eventually resulting in cirrhosis and liver cancer.
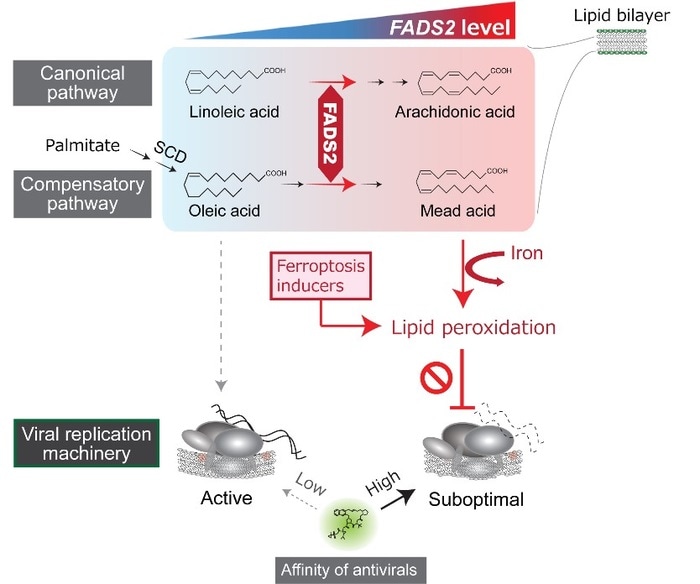
Model showing how fatty acid metabolism triggers lipid peroxidation and regulates hepatitis C virus replication. Production of polyunsaturated fatty acids (PUFAs) is regulated predominantly by FADS2. PUFAs react with reactive oxygen species produced by iron and undergo lipid peroxidation. Lipid peroxidation alters conformation of the viral replication machinery, thereby inhibiting its function. Thus, ferroptosis inducers, drugs that enhance oxidation of PUFAs, increase the efficacy of antiviral drugs. Image Credit: Daisuke Yamane DVM, PhD, Tokyo Metropolitan Institute of Medical Science.
Recent research by scientists from the Tokyo Metropolitan Institute of Medical Science (TMIMS) and Ochanomizu University show that lipid metabolism in liver cells safeguard protection against hepatitis C virus infection. The findings have been published in the Cell Chemical Biology journal.
The observations depict that greater levels of polyunsaturated fatty acids (PUFAs) in liver cells are linked to the resistance of hepatitis C virus infection. The enzyme fatty acid desaturase 2 (FADS2) regulates PUFA synthesis so an increased FADS2 expression results in increased PUFA levels. PUFAs react with reactive oxygen species of iron and undergo oxidative degradation (lipid peroxidation).
Lipid peroxidation takes place on PUFA-enriched membranes where viral replication machinery (RNA replicase) is anchored. When exposed to lipid peroxidation, the viral replicase changes its conformation and disables its capability to replicate the viral genome.
The process that promotes lipid peroxidation and restricts viral replication is quite similar to what occurs during ferroptosis, an iron-dependent form of programmed cell death characterized by increased lipid peroxidation. By enhancing the oxidation of PUFAs using drugs called ‘ferroptosis inducers’, liver cells can limit hepatitis C virus replication, even at low doses of drugs that do not trigger ferroptosis.”
Daisuke Yamane DVM, PhD, Study Co-Author, Chief Researcher, Viral Infection Control Project, Tokyo Metropolitan Institute of Medical Science
The regulatory mechanisms regulating lipid peroxidation are much researched, especially in cancer biology, as specific types of cancer cells are sensitive to ferroptosis. Numerous ferroptosis-inducing drugs with enhanced efficiency and stability have been created as a strategy to treat chemotherapy-resistant cancers. But these drugs do not display antiviral effects.
Our findings suggest that ferroptosis inducers may increase the efficacy of antiviral drugs targeting the viral replicase component. More animal experiments are needed to test this idea, and different ferroptosis-inducing drugs can be examined for efficacy, safety, dosage, and timing.”
Daisuke Yamane DVM, PhD, Study Co-Author, Chief Researcher, Viral Infection Control Project, Tokyo Metropolitan Institute of Medical Science
The research also showed an unusual fatty acid metabolism in cultured liver cells mostly used in labs. Mead acid—normally absent in the human body—is seen in people with low dietary consumption of essential PUFAs, including linoleic acid and a-linolenic acid. The researchers discovered Mead acid in cultured cells in abundance.
Cells cultured in laboratories are fed animal serum as a source of PUFAs but our results suggest that this may not be sufficient for some cell types. Our findings highlight the need for improved cell culture models that recapitulate fatty acid metabolism in the human body. Nonetheless, our data indicate that cellular PUFA synthesis regulates lipid peroxidation, sensitivity to iron-regulated cell death, and hepatitis C virus replication.”
Ikuyo Ichi, PhD, Study Co-Author and Associate Professor, Faculty of Core Research, Natural Science Division, Ochanomizu University
Source:
Journal reference:
Yamane, D., et al. (2021) FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chemical Biology. doi.org/10.1016/j.chembiol.2021.07.022.