The last decade exploited the cell as a powerful tool to perform unexpected tasks by artificial regulation. The cell can function as a promising chemical factory provided the many reactive intermediates developed in the complex metabolic mechanisms and the elusive redox balance assisting cellular homeostasis.
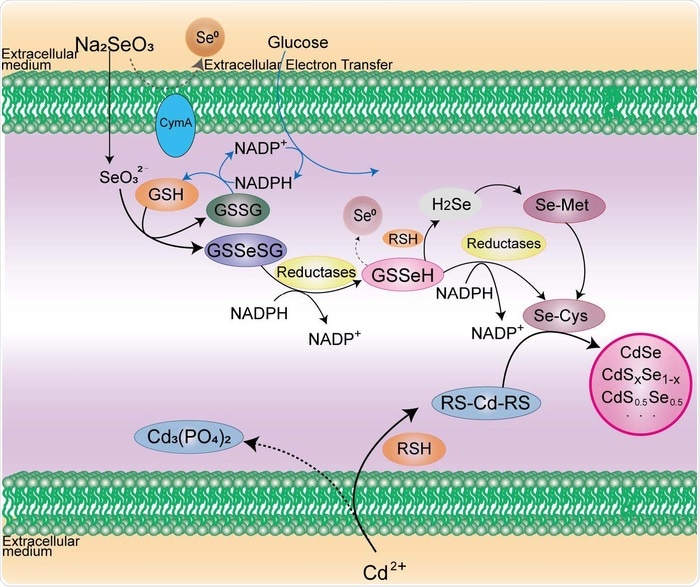
In the case of live-cell synthesis of CdSe QDs, it is essential to produce both reactive Se- and Cd-containing precursors at the proper intracellular location and timepoint by modulating intracellular reductive metabolism of sodium selenite and the detoxification of cadmium ion. Image Credit: © Science China Press.
But it is yet a challenge to drive these reactions and pathways to produce required products as they naturally exist in various spatiotemporal dimensions within the cell. Professor Dai-Wen Pang and their team put forth a concept of “space-time coupled live-cell synthesis of nanocrystals.”
It involves synthesizing nanocrystals by purposefully and accurately coupling a series of intracellular redox reactions and metabolic pathways, in a suitable temporal and spatial sequence in live cells.
The researchers used yeast cells as an example. Fluorescent semiconductor CdSe quantum dots (QDs) with tunable emission wavelengths were produced by coupling the intracellular reductive metabolism of sodium selenite (Na2SeO3) with the induced detoxification of cadmium ion (Cd2+). They appropriately manipulated the glutathione metabolic pathways, to increase the yield of QDs greatly.
This showed the vital role of glutathione during the creation of CdSe nanocrystals. This alluring strategy for the live-cell synthesis of nanocrystals was later expanded to bacterial cells and mammalian cells. Notably, cell-derived microvesicles can also be efficiently labeled in situ with intracellular-synthesized QDs.
Owing to the excellent fluorescent properties, intrinsic high extinction coefficient, and biocompatibility, the intracellular-synthesized QDs, and the QD-containing cells were successfully transformed into nanobioprobes for biodetection, with an electron and energy relay in an artificial photosynthesis system being generated.
Motivated by these processes, cell-free quasi-biosynthesis systems that emulate the intracellular reactions were created to produce various nanocrystals under mild conditions, like alloy nanoparticles, noble-metal nanoparticles, fluorescent and functionalized QDs, and other inorganic nanocrystals. This universal quasi-biosynthesis is more flexible and diversified, which further strengthens the methodology for biosynthesis.
A living cell, as a reservoir of biochemical reactions, can function as an integrated chemical plant where precursor development, nanocrystal nucleation and growth, and functional assembly can be executed precisely by artificial programming. But the current artificial-regulated synthesis utilizes only the tip of an iceberg of metabolic pathways.
We hope to intrigue more researchers to explore new strategies and mechanisms to produce diverse multifunctional crystals and even intricate hetero nanostructures. The concept we proposed will enable researchers to better exploit the unanticipated potentialities of live cells, open a new window for the field of synthetic biology, and shed light on the interdisciplinary studies of biology, chemistry, and medicine.”
Dai-Wen Pang, Professor, Nankai University
Source:
Journal reference:
Liu, A.-A., et al. (2021) Artificial-regulated synthesis of nanocrystals in live cells. National Science Review. doi.org/10.1093/nsr/nwab162.