A novel therapeutic approach inhibits the growth of metastatic tumors in mice by coercing cancer cells into a dormant state—where they cannot proliferate. The observations of the recent research can pave the way for new treatments that inhibit the spread or recurrence of numerous cancer types, including head and neck squamous cell carcinoma (HNSCC) and breast cancer.
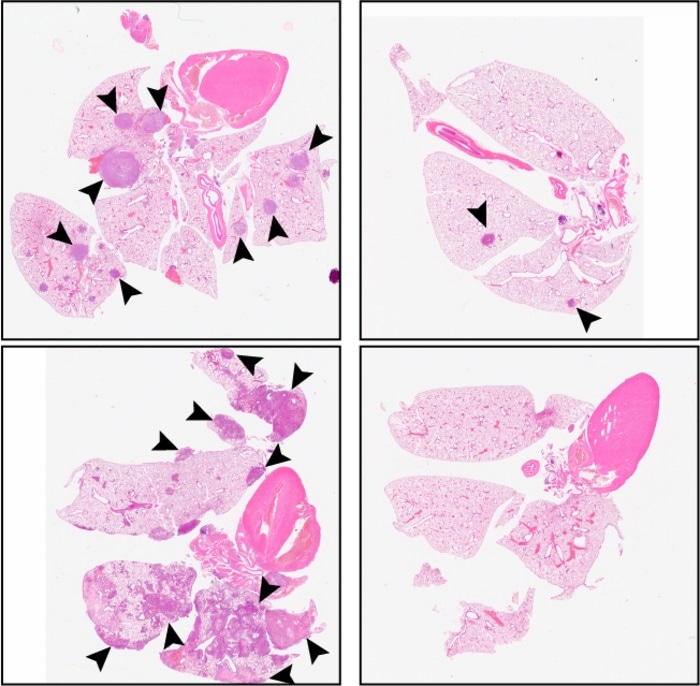
Compared with a control (left panels), C26 treatment (right panels) reduces the number of metastatic tumors in the lungs of mice injected with HSNCC cells. Image Credit: © 2021 Khalil et al. Originally published in Journal of Experimental Medicine.
The research was published on November 23rd, 2021 in the Journal of Experimental Medicine (JEM).
Numerous cancer patients relapse, mostly years or decades following the initial treatment. They develop new tumors that reoccur in the exact location or metastasize (spread) to other body parts.
These secondary tumors are mostly resistant to treatment and are generated from individual tumor cells that remain dormant for long periods before being reactivated to initiate proliferation. Patient relapse can be evaded if scientists identified a means to keep remaining cancer cells in a state of dormancy.
In earlier research, Maria Soledad Sosa from the Icahn School of Medicine at Mount Sinai and Julio A. Aguirre-Ghiso, currently at Albert Einstein College of Medicine, found that the capability of cancer cells to be in a dormant state is regulated by a protein named NR2F1.
This receptor protein is capable of entering the cell nucleus and turns various genes off or on to activate a program that hinders the cancer cells from proliferating. NR2F1 levels are generally low in primary tumors however it is elevated in dormant disseminated cancer cells. NR2F1 protein levels later decline when cancer cells begin to proliferate and form recurrent or metastatic tumors.
We, therefore, thought that activating NR2F1 using a small molecule could be an attractive clinical strategy to induce cancer cell dormancy and prevent recurrence and metastasis.”
Julio A. Aguirre-Ghiso, Albert Einstein College of Medicine
In the current research, Sosa and Aguirre-Ghiso’s group employed a computer-based screening approach to pinpoint a drug, called C26, that activates NR2F1. The scientists discovered treating patient-derived HNSCC cells with C26 enhanced NR2F1 levels and arrested cell proliferation.
The scientists later investigated if C26 could inhibit metastasis in mice. Animals injected with patient-derived HNSCC cells normally develop large primary tumors that disseminate to the lungs after the removal of the original tumor.
Treatment with C26 decreased the size of primary tumors—further doses of C26 entirely blocked the growth of metastatic tumors, after surgery. The rodent’s lungs had a few dormant disseminated cancer cells that were not able to proliferate even after the treatment was discontinued.
Sosa and Aguirre-Ghiso’s group found that by activating NR2F1, C26 pushes cancer cells into a long-lived dormant state characterized by an exceptional pattern of gene activity. Cancer patients whose tumors show a similar pattern of gene activity tend to go longer without relapsing. This indicates that triggering this dormancy program with C26-type drugs can be effective in humans.
Drugs that activate NR2F1 might be particularly useful in breast cancer. NR2F1 is highly enriched in ER-positive tumors when compared to ER-negative tumors, and activating NR2F1 might be able to suppress reawakening of dormant cancer cells kept in that state by anti-estrogen therapies.”
Maria Soledad Sosa, Icahn School of Medicine at Mount Sinai
But, as C26 treatment increases the levels of NR2F1, the approach might also be helpful for other cancers with low levels of receptor protein.
Overall, our study reveals a mechanism-based and rationally designed strategy to exploit NR2F1-activated dormancy as a therapeutic option to prevent metastatic relapse.”
Julio A. Aguirre-Ghiso, Albert Einstein College of Medicine
Source:
Journal reference:
Khalil, B. D., et al. (2021) An NR2F1-specific agonist suppresses metastasis by inducing cancer cell dormancy. Journal of Experimental Medicine. doi.org/10.1084/jem.20210836.