Nanoengineers from the University of California San Diego recently created a new and potentially efficient means to deliver messenger RNA (mRNA) into cells. The new approach involves packing mRNA within nanoparticles that imitate the flu virus—a naturally effective vehicle for delivering genetic material like RNA inside cells.
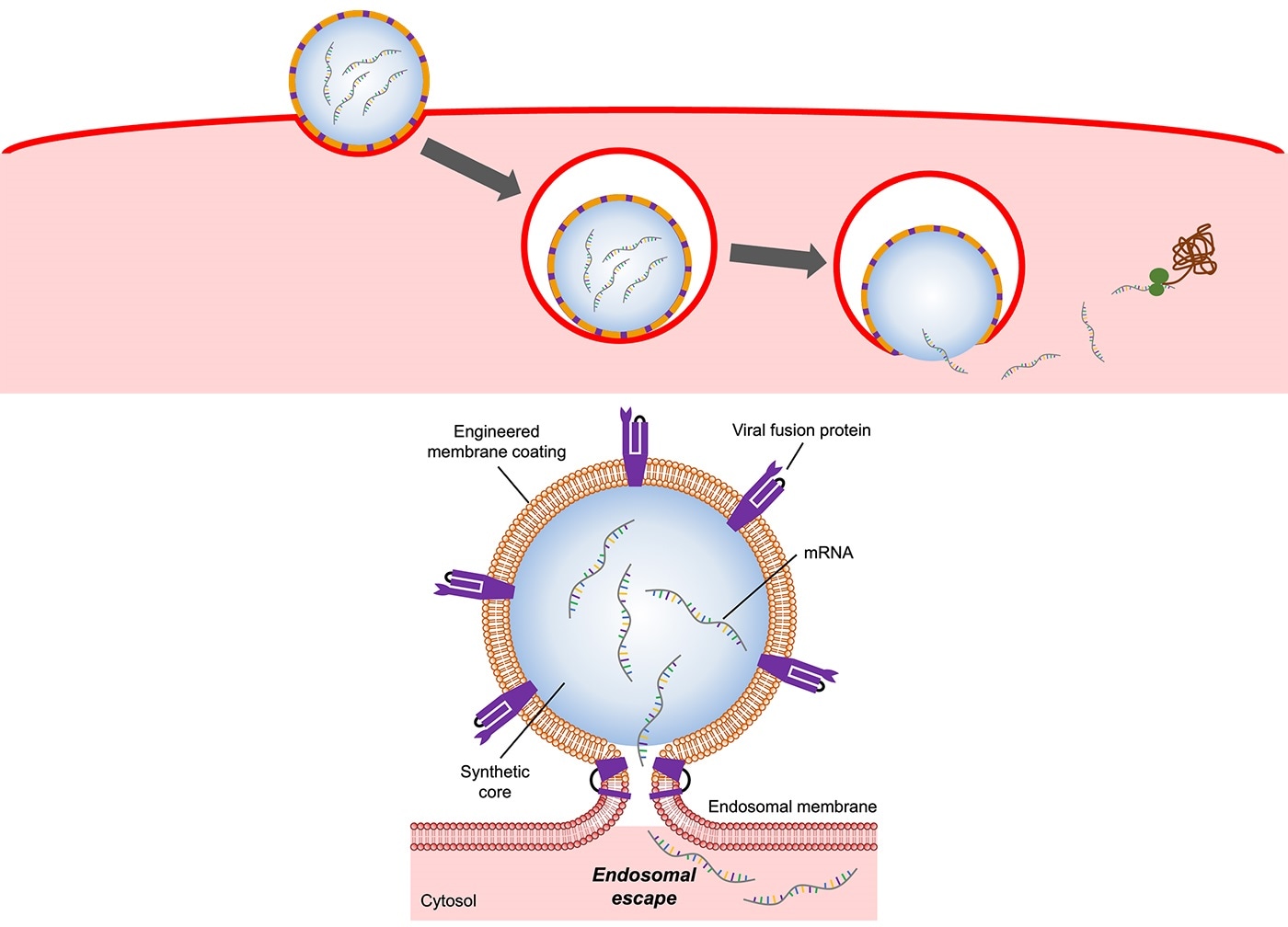
Illustration of a flu virus-mimicking nanoparticle entering and releasing mRNA into a host cell (top). A special protein on the nanoparticle’s surface triggers it to fuse with the endosomal membrane, allowing its mRNA cargo to safely escape into the host cell (bottom). Image Credit: Image adapted from Angewandte Chemie International Edition.
The novel mRNA delivery nanoparticles were detailed in the study published in the journal Angewandte Chemie International Edition.
The study approaches a key challenge in the field of drug delivery—delivering huge biological drug molecules securely into cells and safeguarding them from organelles known as endosomes. These small acid-filled bubbles within the cell function as barriers that entrap and digest bigger molecules that try to enter. For biological therapeutics to perform their function once inside the cell, they require a means to evade the endosomes.
Current mRNA delivery methods do not have very effective endosomal escape mechanisms, so the amount of mRNA that actually gets released into cells and shows effect is very low. The majority of them are wasted when they get administered.”
Liangfang Zhang, Study Senior Author and Professor, Nanoengineering, Jacobs School of Engineering, University of California San Diego
Accomplishing effective endosomal escape will be a game-changer for mRNA therapies and vaccines stated Zhang.
If you can get more mRNA into cells, this means you can take a much lower dose of an mRNA vaccine, and this could reduce side effects while achieving the same efficacy.”
Liangfang Zhang, Study Senior Author and Professor, Nanoengineering, Jacobs School of Engineering, University of California San Diego
It can also enhance the delivery of small interfering RNA (siRNA) into cells, which can be employed in certain forms of gene therapy.
Viruses are better at evading the endosome by nature. The influenza A virus, for instance, has a specific protein on its surface known as hemagglutinin. Hemagglutinin when activated by acid within the endosome induces the virus to fuse its membrane with the endosomal membrane. This opens up the endosome, facilitating the virus to discharge its genetic material into the host cell without getting killed.
Zhang and his group created mRNA delivery nanoparticles that imitate the flu virus’s capability to perform this. To produce the nanoparticles, the scientists genetically engineered cells in the laboratory to express the hemagglutinin protein on their cell membranes.
The researchers later separated the membranes from the cells, shattered them into small pieces, and coated them onto nanoparticles produced from a biodegradable polymer that was pre-packed with mRNA molecules.
The end product is a flu virus-like nanoparticle that is capable of getting inside a cell, shattering the endosome, and releasing its mRNA payload to perform its function—instruct the cell to generate proteins.
The scientists tested the nanoparticles in mice. The nanoparticles were filled with mRNA encoding for a bioluminescent protein named Cypridina luciferase. They were administered to the mice through intravenous injection and the nose—the mice were made to inhale droplets of a nanoparticle-containing solution applied at the nostrils.
The scientists pictured the noses and assayed the blood of the mice and identified a substantial amount of bioluminescence signal. This was proof that the flu virus-like nanoparticles efficiently delivered their mRNA payloads into cells in vivo.
Currently the scientists are investigating their system for the delivery of siRNA and therapeutic mRNA payloads.
Source:
Journal reference:
Park, J. H., et al. (2021) Virus-Mimicking Cell Membrane-Coated Nanoparticles for Cytosolic Delivery of mRNA. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.202113671.