A thread-like highway, called microtubule tracks, allows motor proteins to transport cargo inside neurons.
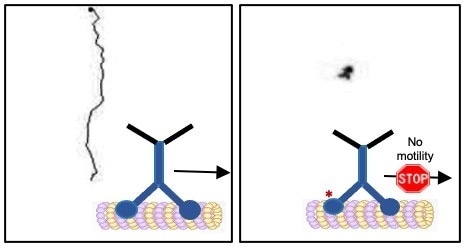
A study describes how an enzyme called GSK3β can act as a stop switch for a motor protein called kinesin 1. The dark line in the left panel shows the trajectory of a kinesin 1 motor protein with normal movement. The dark spot in the right panel shows the trajectory of a kinesin 1 motor protein whose motion has been stalled. Image Credit: Rupkatha Banerjee, adapted from a figure published in Development in a December 23rd, 2021 article by Banerjee et al.
This small highway system is critical for maintaining the health of neurons: When communication flows smoothly, vital materials can reach far-flung parts of the cells where they are needed. When the system fails, it can obstruct cellular function and even cause cell death.
Researchers have discovered a new traffic-control gadget. They further revealed how an enzyme called GSK3β can operate as a stop switch for a type of motor protein called kinesin 1. The study was published in the journal Development.
Our publication details how GSK3β attaches a molecular tag to kinesin 1 motors, which causes the motors to stop without detaching from microtubule tracks. We are super excited, since now we know how to control the ‘engine’ while it is moving on a track.”
Shermali Gunawardena PhD, Study Senior Author and Associate Professor, Biological Sciences, University at Buffalo College of Arts and Sciences
Rupkatha Banerjee, PhD, the study’s first author and a postdoctoral research associate at Scripps Research in Florida commented, “Transport of cargoes by motors is a tightly coordinated process, and yet the molecular mechanisms that control these ‘engines’ along the microtubule tracks remain largely unknown.” Banerjee completed her doctorate in biological sciences at UB.
“Our work provides an in-depth understanding of how the enzyme GSK3β acts as a key regulator of the kinesin 1 motor. Specifically, we have identified a precise site on kinesin 1 that is modified by GSK3β,” says Banerjee.
“Using molecular biology, in vitro analysis and fly genetics, coupled with in vivo imaging techniques, we were able to tease out the mechanistic details by which disruption of this particular site impacts motor movement and motor attachment to cargoes or microtubule tracks in a whole organism,” Banerjee adds.
The discoveries, which were based on laboratory tests, including some in fruit fly larvae neurons, could pave the way for future study into stopping motors as a disease treatment strategy. One possible example, according to Gunawardena, is cancer.
In cancer, cells are rapidly dividing, and motors are involved in this. So if you can stop the motors, you can impact this continuous division of cells.”
Shermali Gunawardena, PhD, Associate Professor, Biological Sciences, University at Buffalo College of Arts and Sciences
In a different perspective, Gunawardena also notes that, “In some neurodegenerative diseases, you see blockages of cargo within neurons because things are getting stuck on the road. If we can control the motors and stop them, maybe we can help to clear the track and get rid of these blockages.”
She further explains, “In parts of California, at rush hour, you have traffic lights that only let so many cars in at a certain time to prevent the highway from getting too full, which would slow down traffic and cause traffic blocks. Perhaps we can also apply this concept in neurons, too, if we can control motors by turning them on or off.”
Piyali Chakraborty, an MS graduate of UB’s neuroscience program, and Michael C. Yu, PhD, associate professor of biological sciences at UB, were co-authors of this study.
In addition to describing how GSK3β can stop kinesin 1 motors, the study looked at other factors of the enzyme’s connection with the motors, with the findings confirming the notion that GSK3β plays a crucial role in fine-tuning kinesin 1 motor movement within the neurons of a living organism.
This publication emphasizes fine-tuning of motor function as a potential approach for restoring transport defects that contribute to neurodegeneration and cancer.”
Rupkatha Banerjee PhD, Study First Author and Postdoctoral Research Associate, The Scripps Research Institute
Source:
Journal reference:
Banerjee, R., et al. (2021) A stop or go switch: glycogen synthase kinase 3β phosphorylation of the kinesin 1 motor domain at Ser314 halts motility without detaching from microtubules. Development. doi.org/10.1242/dev.199866.