Pancreatic cancer is a unique, elusive, and fatal malignancy, with only approximately a 10% five-year survival rate. If cancer has spread, the survival rate drops to 3%. And, there are very limited treatment options available.
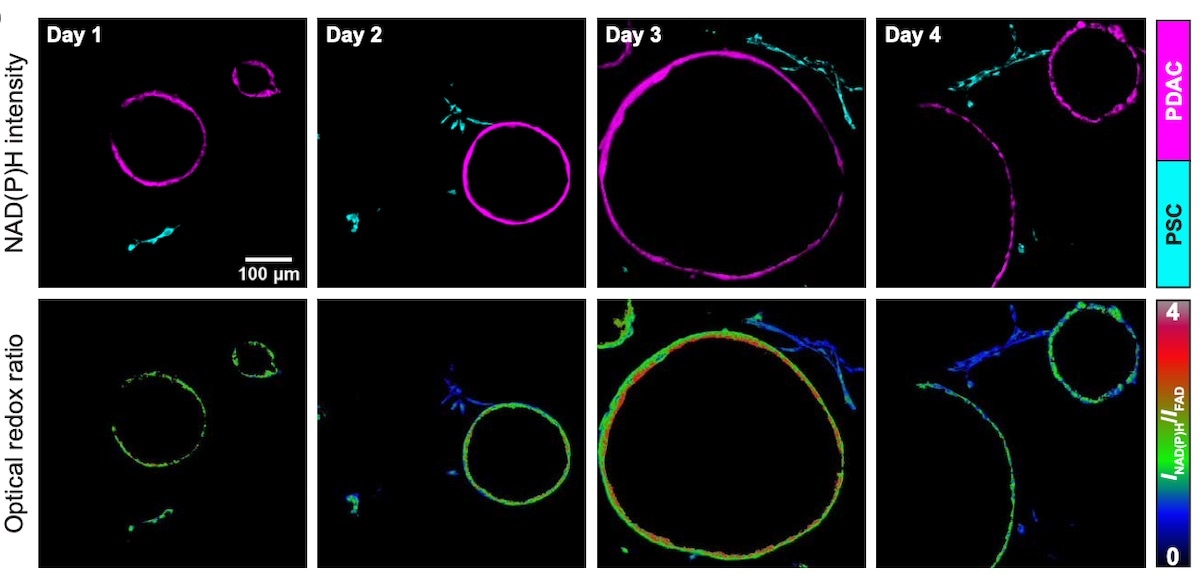
Representative NAD(P)H fluorescence intensity images of pancreatic cancer cells cultured as 3D organoids (shown in magenta) and PSC non-cancer cells (shown in cyan) from cocultures obtained on days 1 through 4 (top). The corresponding optical redox ratio from the same images is also shown (bottom). Image Credit: Morgridge Institute for Research.
It’s one of the scarier cancers because once you discover that a person has pancreatic cancer, it’s often too late because there are no symptoms. The survival rate is so low because at that point, you can’t really do anything.”
Rupsa Datta, Morgridge Institute for Research
Rupsa Datta is an assistant scientist at the Skala lab.
The Skala lab studies the metabolic activity that contributes to tumor growth using enhanced optical imaging in the aim of developing novel medicines and treatments based on a better knowledge of the tumor microenvironment.
Datta and colleagues show how cancer cells may hijack the metabolic function of specific non-cancer cells in the pancreas to drive tumor growth in a study published in the journal Science Advances.
The extracellular matrix (ECM), a network of chemicals that helps give structure for the cells that make up an organ like the pancreas, is an important aspect of the tumor microenvironment when cancer cells start to grow.
Immune cells, fibroblasts, and organ-specific supporting cells such as pancreatic stellate cells (PSCs) are examples of non-cancer cells found in the ECM.
Datta states, the interactions between cancer cells and these PSCs are vital to the tumor’s growth and survival.
The cancer cells can recruit those non-cancer cells to work for them. It’s like they’re under the cancer cell’s spell. They will bring them nutrients and other things so that the cancer cell can survive.”
Rupsa Datta, Morgridge Institute for Research
The researchers created an organoid model, a 3D cell culture environment within a gel-like matrix structure that more accurately resembles the biology of a living organ or tumor, by combining pancreatic cancer cells with PSCs.
Researchers can use optical metabolic imaging to see and evaluate metabolic interactions between cells in real time. The method is non-invasive and label-free, meaning it relies on the cells’ own autofluorescence as a readout rather than introducing extra chemicals that may harm them.
The reduction-oxidation state of the cells, or redox state, fluctuates as electrons are transported between molecules inside a cell to help it grow and divide and is used to assess changes in metabolism.
Datta explains, “We found that when these non-cancer cells are present, the cancer cell redox state becomes more oxidized and moves toward proliferation. When the PSCs were touching the cancer cells, they were becoming more reduced.”
Cancer cells may be breaking their redox constraint of cell proliferation by engaging directly with PSCs and using the metabolic process to boost cancer development, according to this transfer between cells.
It is still unclear how the physical interaction between these cells results in redox state changes. Datta, on the other hand, believes that a better understanding of these interactions may lead to targeted drugs that will stop cancer cells from proliferating and tumors from growing.
With financing from the nonprofit group Stand Up to Cancer (SU2C), the Skala Team worked closely with cancer scientist Matt Vander Heiden and his lab at the Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology (MIT) on this work.
Most of our collaborators are in Wisconsin, but this was a long-distance collaboration, so because of the grant, I could travel to their lab and they could come here, too. It was a very interesting project because we have expertise in different fields. And we both need to answer these questions.”
Rupsa Datta, Morgridge Institute for Research
While the Skala Lab pioneered the innovative imaging techniques used to get these metabolic measures, the Vander Heiden Lab was crucial in establishing the organoid model and putting the results into biological context and significance in the cancer area, adds Datta.
“I’m hoping this paper will show the power of our technique. Though, if more cancer labs adopted it, they would still need to collaborate with labs like ours with imaging expertise. But we love to collaborate!” Datta concluded.
Source:
Journal reference:
Datta, R., et al. (2022) Interactions with stromal cells promote a more oxidized cancer cell redox state in pancreatic tumors. Science Advances. doi.org/10.1126/sciadv.abg6383.