Researchers currently understand that the microorganisms that dwell in human intestines—the microbiome—have a wide range of effects on human health. Inflammatory bowel disease (IBD), immunological disorders, neurological illnesses, cancer, and other diseases are thought to be affected by the balance of competing microbial species.
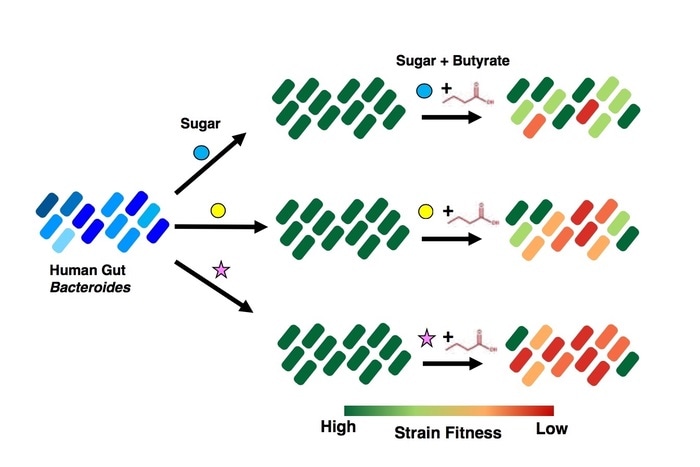
This schematic illustrates the growth or “fitness” of various Bacteroides species exposed to different sugars, alone and with the bacterial metabolite butyrate. The strains in green on the right are the most fit and best able to tolerate butyrate, while those shown in red die off. The fitness profiles vary from species to species and from sugar to sugar. Image Credit: Boston Children’s Hospital.
Sugars in daily diets, in response, have an impact on this equilibrium, determining which microorganisms survive and which do not. Furthermore, microbes produce dozens of distinct metabolites that have an impact on each other’s survival—as well as human health.
Seth Rakoff-Nahoum MD, PhD, of Boston Children’s Hospital’s Divisions of Infectious Diseases and Gastroenterology, has published a new study that lays out some critical elements in determining how these complicated elements come together. Bacteroides, the most common Gram-negative bacteria in the human intestine, was his focus. On February 3, the findings were published in the journal Cell.
We need to learn how each species and strains of the gut microbiome, in particular Bacteroides species, survive in our guts if we want to enhance them or diminish them. Gut bacteria compete to use the undigested sugars in our diets as their energy source. Some have half their genome dedicated to better using these sugars.”
Seth Rakoff-Nahoum, Divisions of Infectious Diseases and Gastroenterology, Boston Children’s Hospital
A three-way interaction
Rakoff-Nahoum and colleagues investigated how chemicals in the gut affect Bacteroides proliferation. Butyrate, a short-chain fatty acid that plays an important role in the host, including immune system growth, stood out as an inhibitory metabolite to specific Bacteroides. When the researchers cultured Bacteroides in different sugars from the diet of humans, they discovered that the antimicrobial impact of butyrate varied depending on which sugar each strain used.
If you take any two strains and grow each in Sugar X, in the presence of butyrate, Strain A will be killed dead in its tracks while Strain B grows as if butyrate was not even there. With Sugar Y, we see the opposite: Strain B is now is killed by butyrate, while Strain A is unaffected.”
Seth Rakoff-Nahoum, Divisions of Infectious Diseases and Gastroenterology, Boston Children’s Hospital
Why does butyrate kill some Bacteroides but not others? A third dimension was discovered in the study: genetic factors. Bacteroides strains differed in their susceptibility to butyrate due to minor differences in genes regulating Coenzyme A metabolism.
We initially thought these genes would be very similar among all bacteria, as they perform basic cellular functions. But the Bacteroides change them, likely to help them deal with the special environment in our gut.”
Seth Rakoff-Nahoum, Divisions of Infectious Diseases and Gastroenterology, Boston Children’s Hospital
This work is only the first step. “We want to explore how combinations of metabolites and specific glycans in the diet can help us shape the microbiome for conditions in which we think the microbiome is important, such as IBD, allergy, and cancer,” he adds.
Source:
Journal reference:
Park, S.-Y., et al. (2022) Strain-level fitness in the gut microbiome is an emergent property of glycans and a single metabolite. Cell. doi.org/10.1016/j.cell.2022.01.002.