A signaling mechanism that enables bacteria like Salmonella to escape destruction by the immune system of the host was discovered by the scientists at UC Davis.
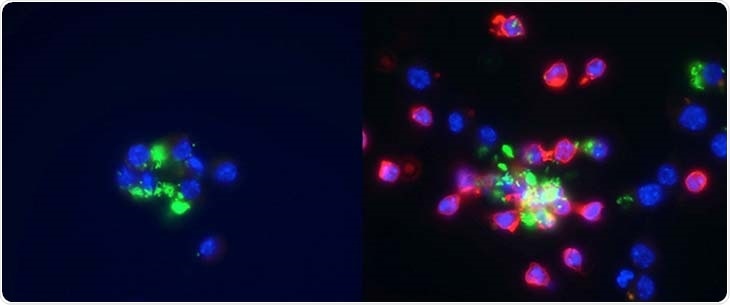
Left: macrophages are infected with Salmonella, shown in green. Right: neutrophils, shown in red, are added to macrophages infected with Salmonella, shown in green. Image Credit: University of California, Davis
Macrophages, a type of immune cell, can be infected by intracellular bacteria like Salmonella. The death of the host macrophage is triggered by Salmonella. Further, the bacteria trick other immune cells into delivering them to another macrophage safely, instead of destroying them.
The Salmonella outmaneuvers host defenses by using one of our best weapons and turning it against us. It is kind of ingenious.”
Andreas Bäumler, Study Senior Author, Professor and Vice-Chair of Research, Department of Medical Microbiology and Immunology, UC Davis School of Medicine
The study was done on mice tissue cultures and published in the journal Cell Host & Microbe.
Pathogens hiding in immune cells
A part of the immune system, also known as the complement system, is responsible for cleaning up pathogens, along with bacteria. It either directly destroys the pathogen or aims it for destruction through other immune cells.
Neutrophils are immune cells, complementing system recruits to handle bacteria. “Their job is to kill bacteria. If you scrape your skin, you survive because the neutrophils take care of the random bacteria that come in,” Bäumler elaborated.
Macrophages are another type of immune cell that washes up lifeless and dying cells. They engulf bacteria and other cellular remains actively, but they are not as efficient at killing bacteria as neutrophils are.
Bacteria like Salmonella live inside macrophages in a vacuole, which is enclosed by a membrane, or a compartment. Staying inside the macrophage enables them to evade detection by other immune cells, like bacteria-killing neutrophils.
Intracellular bacteria like Salmonella should periodically get a new host cell as macrophages can live only about 30 days. Furthermore, they have to do it without getting identified and damaged by the immune system.
The scientists discovered that the macrophage-infected Salmonella bacteria use particular weapons, called virulence factors, to make holes in the membranes of the vacuole and the macrophage to stimulate the death of macrophage, eventually, opening channels to the outside.
These holes enable the bacteria to turn on the complement system and produce a “find-me” signal to attract neutrophils to identify bacteria trapped in dying macrophages.
The neutrophil engulfs the Salmonella and the dead macrophage in a process called efferocytosis when it comes to cleaning up the lifeless macrophage with the Salmonella present inside.
Salmonella is shielded by dead macrophages from the antimicrobial properties of the neutrophil, enabling the bacteria to survive.
Therefore, Salmonella takes over the key aspects of the host immune system—complement system and neutrophils—to stay within the infected host.
When the pathogen triggers the host complement system by virulence factor-induced perforation within a host macrophage—which generates a signal for efferocytosis—the system doesn’t work anymore. Neutrophils become a safe site for the Salmonella.”
Hirotaka Hiyoshi, Study Lead Author and Assistant Professor, Department of Bacteriology, Nagasaki University
Hiyoshi was formerly an assistant project scientist in the UC Davis Department of Medical Microbiology and Immunology.
Understanding the mechanism may help future treatments
Salmonella is one of the types of intracellular bacteria that can cause disease in people. Other pathogens that live within cells include Listeria monocytogenes, Brucella abortus, Chlamydia trachomatis, Mycobacterium tuberculosis, and Coxiella burnetii.
The scientists also established that Brucella uses the same mechanism as Salmonella to avoid the host immune system, proposing that this virulence strategy might be shared among bacterial pathogens that are capable of surviving inside host cells.
Although few of these bacterial infections may resolve by themselves, they mostly need antibiotic treatment. Antibiotic resistance is anticipated to considerably increase in the future, implying that they might not be effective against intracellular pathogens anymore.
Multi-drug resistance is on the rise, and by 2050, multi-drug resistant bacteria are projected to be the number one cause of death. This is why we need to understand these diseases to find a vulnerability in their strategies. Once you understand the mechanism, you may be able to develop a new target treatment.”
Andreas Bäumler, Study Senior Author, Professor and Vice-Chair of Research, Department of Medical Microbiology and Immunology, UC Davis School of Medicine
Source:
Journal reference:
Hiyoshi, H., et al (2021) Virulence factors perforate the pathogen-containing vacuole to signal efferocytosis. Cell Host & Microbe. doi.org/10.1016/j.chom.2021.12.001.