Reviewed by Danielle Ellis, B.Sc.Feb 14 2022
In the early days of SARS-CoV-2 infection in the lungs, what happens at the single-cell level?
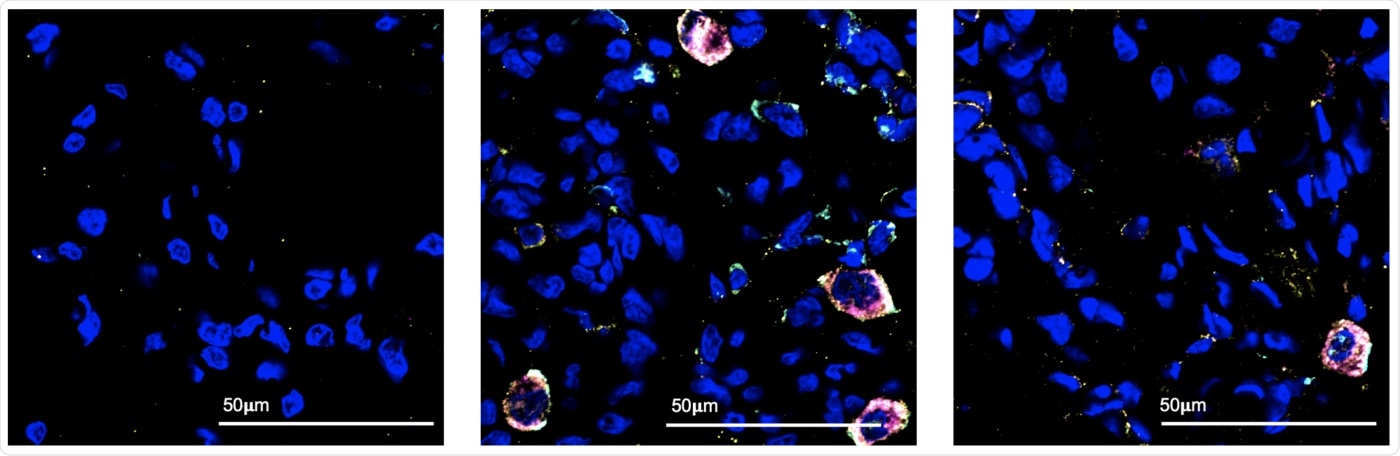
Using fluorescent markers, Texas Biomed researchers and colleagues identified the cell populations active in the lung in the first few days of COVID-19 infection. Under the microscope, they observed immune cells called macrophages (the large multicolored circles) responding to SARS-CoV-2 infection, at three days following infection (center). These macrophages were not present in the lung before infection (left) and most were no longer detected two weeks after infection (right). Blue spots are nuclei of normal lung cells. Image Credit: Texas Biomed
In collaboration with Washington University in St. Louis, researchers from Texas Biomedical Research Institute and Southwest National Primate Research Center (SNPRC) have determined what immune cells are present in the lungs during the early stages of SARS-CoV-2 infection, and what some of those cells are doing to combat the virus. The findings, which were published in the journal Nature Communications, will aid in the development of future COVID-19 therapies.
This is the most detailed analysis of early SARS-CoV-2 infection to date thanks to the latest single-cell sequencing technologies, and animal models developed at Texas Biomed and SNPRC.”
Deepak Kaushal PhD, Study Senior Author and Director, Southwest National Primate Research Center, Texas Biomedical Research Institute
The findings have shed light on the main puzzle surrounding the COVID-19 pandemic: the significance of Type I Interferons, a type of signaling protein (IFN). Interferon molecules operate as sentries or warning bells to other cells during viral infections, blasting “intruder alert!” so they can strengthen their defensive systems.
However, other studies have found that SARS-CoV-2 has a reduced Type I Interferon response, allowing the virus to propagate more easily. At the same time, severe COVID-19 has been associated with uncontrolled interferon “cytokine storms.”
Scientists have been attempting to determine if interferon fights SARS-CoV-2 or is dysregulated in some way, particularly early in infection. Clarifying this is critical for designing therapies that try to prevent harmful inflammation caused by high interferon activity without disrupting the beneficial functions of the protein.
According to the new study, interferon plays a critical role in viral clearance by signaling other immune cells called macrophages, to look for and destroy the virus. Macrophages are similar to Pac-Man as they gobble up virus-infected cells.
Our analysis shows there is a massive population of macrophages in the lungs at day three after infection, amounting to 80 to 90 percent of all cells in the airways at that moment. We can also tell by looking at the genes that are activated in those macrophages, that they are specifically responding to an interferon signal.”
Dhiraj K. Singh PhD, Study First Author and Staff Scientist, Texas Biomedical Research Institute
Single-cell RNA sequencing, the most recent and highest-resolution genetic sequencing method, was used to make the discovery. More than 170,000 individual cells’ gene expression patterns were sequenced by scientists. These profiles, showing which genes are switched on or off, reveal the identity of the cell and the cell activity at any given time.
To analyze and understand the huge volumes of raw, high-resolution data, Singh collaborated closely with Shabaana Khader PhD, and Maxim Artyomov PhD, and their Washington University in St. Louis teams.
“RNA sequencing has been around for 10 years, but it averages out gene expression activity for an entire tissue. In contrast, the latest single-cell RNA sequencing can tell you what genes are on and off in say, B cells versus T cells. It is so much more specific,” stated Singh.
However, without animal models, the data collection would not have been possible in the first place. Scientists collect what amounts to a lung wash from rhesus macaques at important time intervals to acquire a picture of the lung environment before, during, and after infection.
Singh explains, “Often, by the time people go to the clinic when they are sick, the virus is already well established. Primates are the only model where we can actually look at those early acute responses.”
The San Antonio community rallied behind Texas Biomed and SNPRC during the outset of the COVID-19 pandemic, giving more than $5 million in a week to assist the institute’s efforts to produce animal models for COVID-19 research, according to Kaushal. Animal models have since been used to test vaccinations and medicines, such as the Pfizer-BioNTech vaccine and Regeneron’s monoclonal antibody cocktail.
This new research is a continuation of the animal model that we generated, which would not have been possible without the generous contribution of the San Antonio community and ongoing support from the National Institutes of Health’s Office of Research Infrastructure Programs. It shows once you’ve established a model, what is possible. You can really advance knowledge in the area.”
Deepak Kaushal, Study Senior Author, Ph.D, Director, Southwest National Primate Research Center, Texas Biomedical Research Institute
Singh recently got funding from the San Antonio Medical Foundation to investigate what occurs when more interferon is administered to the lungs, as well as to compare the results to other interferon-blocking research now underway.
If the investigations show interferon’s favorable impact in marshaling macrophages early in infection, interferon-based treatments for COVID-19 might be developed. These have been tested for various disorders and may be ready for clinical trials soon.
“In my opinion, the potential applications don’t stop at COVID-19. It’s quite likely that the next pandemic in the next 10 years or so will be caused by another coronavirus. So having all this knowledge is going to be very critical,” Kaushal concluded.
Source:
Journal reference:
Singh, D. K., et al. (2022) Myeloid cell interferon responses correlate with clearance of SARS-CoV-2. Nature Communications. doi.org/10.1038/s41467-022-28315-7.