In Finland, about 700 people are identified with diffuse large B-cell lymphoma (DLBCL) every year. It is the most common cancer, originating from lymphocytes, immune system cells. The disease is cured with the objective of acquiring the whole remission and avoiding recurrence, however, one-third of the patients go through progression with a dismal prognosis.
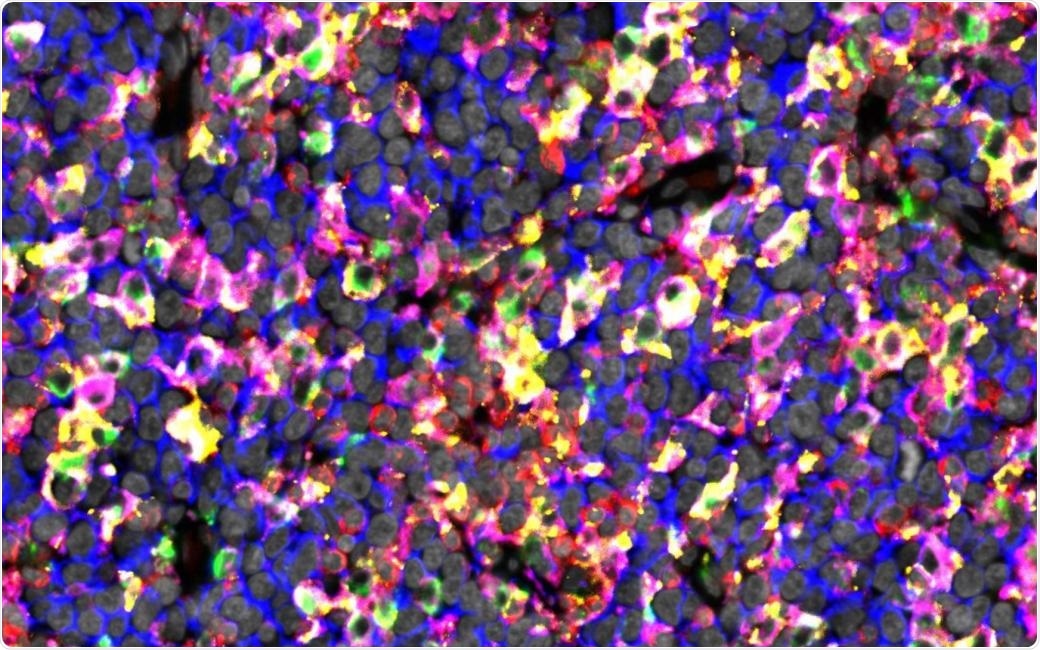
The figure shows immunostaining of lymphoma tissue rich in checkpoint proteins expressing macrophages. Image Credit: University of Helsinki.
DLBCL is a heterogeneous disease—one of the challenges in this treatment—and enhanced instruments are required for categorizing the patients for sufficient treatment.
When the success of immunotherapies in Hodgkin lymphoma and solid tumors is considered, tumor-infiltrating immune cells are promising therapeutic targets in DLBCL as well. These checkpoint blockage treatments can aid the immune system of the body to acknowledge and fight against cancer cells.
However, the efficiency of checkpoint blockage treatments against DLBCL has been modest so far. This is primarily because the mechanisms of immune cells’ and lymphoma cells’ communication with each other have not been known. A better understanding would aid in informing strategies for the immunotherapies’ use.
Decoding the composition of tumor-infiltrating immune cells
Currently, scientists at the University of Helsinki and Helsinki University Hospital Comprehensive Cancer Centre have interpreted the heterogeneity of tumors that are infiltrating immune cell composition in DLBCL.
Alongside genomic alterations in the tumor cells, the disease development is affected by the tissues around cancer cells, which is the lymphoma microenvironment. It includes extracellular stroma, blood vessels, and immune cells, along with T lymphocytes and macrophages.
The study was recently published in Clinical Cancer Research. It examined the interplay between the immune cells present in the lymphoma microenvironment. By using new imaging methodologies, advanced data analyses, and gene expression profiling, the researchers defined lymphoma infiltrating T cells and macrophages, along with their spatial communication at the single-cell level in 178 lymphoma samples.
The results were correlated with patient survival and demographics. The results were verified in two external cohorts that comprised a sum of 633 patients.
Clinically meaningful interplay between immune cells
Researchers discovered that depending on the content of the immune cell, DLBCL can be categorized into inflamed and noninflamed subtypes. These are defined by high and low immune cell contents, respectively. DLBCL patients with an increased T cell content but a decreased macrophage content had favorable survival. Nevertheless, inflamed subtype translated to inferior result when macrophages expressed immune checkpoint molecules.
They also found that some checkpoint molecules are developed on macrophages when they communicate with T cells, which suggests that checkpoint molecules are vital in the interplay between macrophages and T cells.
The results expand previous studies, which have majorly concentrated on defining the lymphoma microenvironment depending on gene expression. The findings show that the interplay among immune cells present in the lymphoma microenvironment is clinically important.
In specific, the results pinpoint the extreme effect of checkpoint molecule expressing macrophages on the T-cells’ anti-tumoral activity. The results also imply that checkpoint blockage treatment should be examined in patients in clinical trials with inflamed DLBCLs and checkpoint molecule’s high proportions that express macrophages. In the long run, this could convey a more precise way to categorize DLBCL patients for immunotherapies.
Source:
Journal reference:
Autio, M., et al. (2022) Clinical Impact of Immune Cells and Their Spatial Interactions in Diffuse Large B-Cell Lymphoma Microenvironment. Clinical Cancer Research. doi.org/10.1158/1078-0432.CCR-21-3140.