Reviewed by Danielle Ellis, B.Sc.Mar 2 2022
Scientists have observed for the very first time that when insulin-producing cells in the pancreas are attacked by T lymphocytes during the evolution of Type 1 Diabetes (T1D), the cells that line the pancreatic duct remodel themselves in an attempt to dampen autoimmune T cell responses. The study was published on February 28th, in the journal Nature Metabolism.
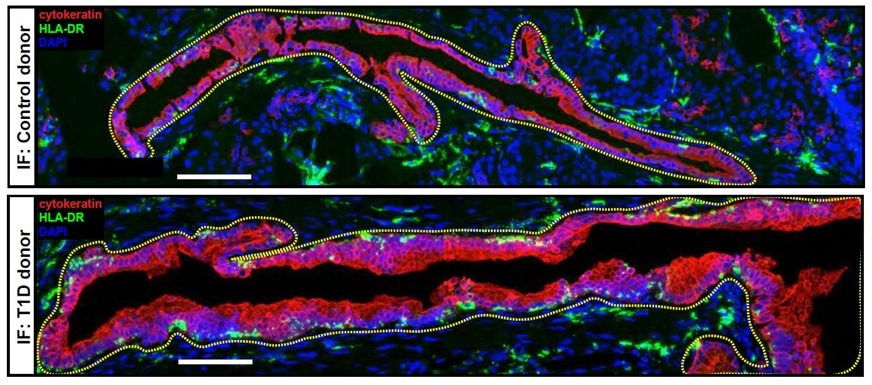
A microscopic image taken from the pancreas of a T1D donor (top) and Control donor (bottom) displaying HLA-DR+ cytokeratin+ cells labeled by immunofluorescence (IF), a technique for determining the location of an antibody in tissues by reaction with an antibody labeled with a fluorescent dye. Image Credit: University of Pennsylvania
The first events that occur in a patient heading towards Type 1 Diabetes, the events that trigger autoimmunity, have been difficult for researchers to pin down because of our inability to biopsy the pancreas, and the fact that clinical diagnosis is only made once massive beta cell destruction has occurred.”
Golnaz Vahedi, PhD, Study Senior Author and Associate Professor, Genetics, University of Pennsylvania
Vahedi was also a member of the Institute for Diabetes, Obesity, and Metabolism at the Perelman School of Medicine at the University of Pennsylvania.
“That is why it is so important to develop a better understanding of the earliest molecular events in T1D pathogenesis, so we can uncover more about biomarker identification and disease prevention,” he said.
Autoimmune illnesses, which affect up to 23.5 million people in the United States, are caused by the immune system attacking and destroying healthy organs, tissues, and cells. Rheumatoid arthritis, inflammatory bowel disease, and T1D are among the more than 80 forms of autoimmune illnesses.
T lymphocytes target and damage insulin-secreting pancreatic beta cells in T1D, resulting in the pancreas ceasing to produce insulin—the hormone that regulates blood glucose levels.
Although it might be an ultimately unsuccessful attempt of the pancreas to limit the adaptive T cell response responsible for destroying beta cells, this finding that the ductal cells are capable of playing this suppressive role towards autoimmune T cell responses is unprecedented.”
Klaus Kaestner, PhD, Study Co-Senior Author and Thomas and Evelyn Suor Butterworth Professor, Genetics, University of Pennsylvania
“Our study shows that these cells, which had never previously been linked to immunity, may change themselves to protect the pancreas,” he added.
The Human Pancreas Analysis Program (HPAP) was founded in 2016 and is funded by a $28 million investment from the National Institutes of Health, with significant contributions from Penn, the University of Florida, and Vanderbilt University.
The HPAP started gathering pancreatic tissues from hundreds of deceased organ donors detected with T1D. The program is co-directed by Kaestner and Ali Naji MD, PhD, the J. William White Professor of Surgical Research.
Since many T1D patients have Glutamic Acid Decarboxylase (GAD)—beta cell autoantibodies—in their bloodstream years before clinical diagnosis. HPAP also takes blood samples from autoantibody-positive donors who are at risk of having T1D but have not yet been diagnosed.
Our study took those quality tissue samples and created high-resolution measurements of millions of cells from patients at various stages of T1D progression, resulting in a single-cell atlas of pancreatic islets.”
R. Babak Faryabi, PhD, Study Co-Senior Author and Assistant Professor, Pathology and Laboratory Medicine, University of Pennsylvania
Faryabi is also a key member of Penn’s Epigenetics Institute.
For patients with or at potential danger for T1D, blood tests to assess for GAD levels are widespread, and doctors use it as a screening tool. Another discovery of this research is a fresh insight into what is going on at a molecular scale in the pancreas and how it relates to the GAD test results.
Naji, a study co-senior author says, “Our study is the first to show that even when a person is not clinically considered to have T1D, high levels detected in their GAD test indicate large-scale transcriptional remodeling of their beta cells.”
“It solidifies to clinicians to closely monitor patients with increasing levels of GAD, as we now know what cellular and molecular changes are in motion in relation to those levels,” he added.
Although it is unclear whether these transcriptional shifts contribute to or are repercussions of disease pathogenesis, the invention of molecular phenotypic changes in pancreatic cells of autoantibody-positive individuals contributes to the understanding of initial pancreatic changes in T1D and sets the stage for future research.
Source:
Journal reference:
Fasolino, M., et al. (2022) Single-cell multi-omics analysis of human pancreatic islets reveals novel cellular states in type 1 diabetes. Nature Metabolism. doi.org/10.1038/s42255-022-00531-x.