Getting from point A to point B might be difficult, especially for mobile cells. By taking a closer look at one of the major players—the pseudokinase ILK, an international collaboration of scientists from the Heidelberg Institute for Theoretical Studies (HITS) and the University of Helsinki has focused on the biochemical and biomechanical mechanisms behind cell migration.
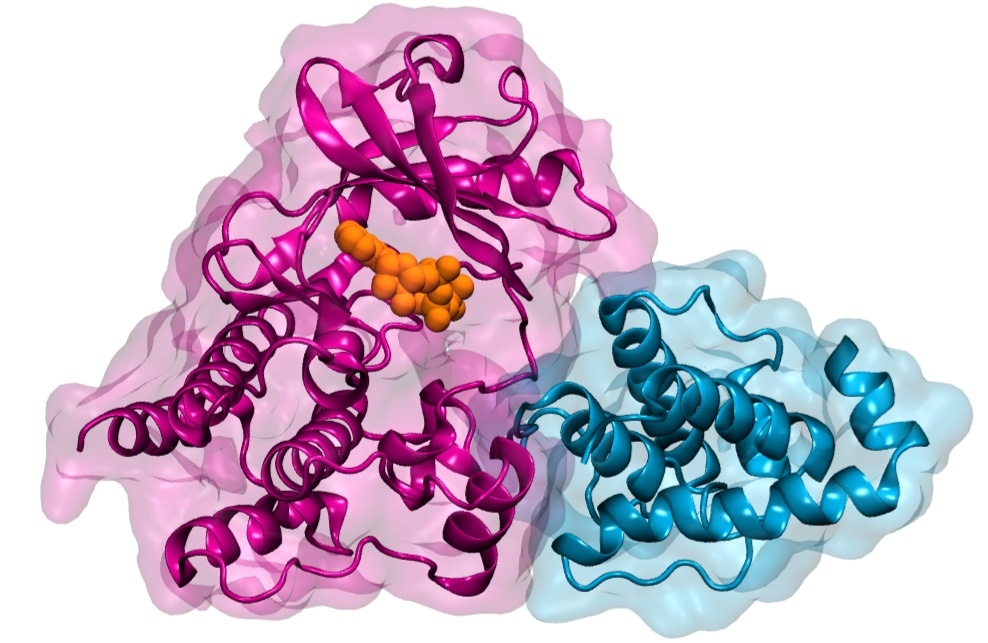
Illustration of Integrin-linked Kinase (ILK) (pink) binding to its partner parvin (blue). Both proteins are represented as a ribbon diagram overlayed with the proteins´ outlines. The small molecule ATP bound to ILK is represented as orange spheres. Image Credit: Heidelberg Institute for Theoretical Studies.
The findings of their research add to the knowledge of this fascinating protein. The results are published in the Proceedings of the National Academy of Sciences (PNAS).
While the bulk majority of cells stay relatively stationary throughout their lives and only travel short distances, a few specialized cells—including such immune cells or the so-called fibroblasts employed by the researchers in this study—require the freedom to move freely and fast in order to perform their functions, such as during wound healing.
They must achieve the proper balance between structural support and flexibility by perceiving and reacting to a wide range of biochemical and mechanical cues from their adjacent cells and the matrix to which they attach, whether they are quick or weak, inert or nimble.
This signal transmission between the cell and the matrix is mediated by large protein complexes. Integrin-linked kinase is a key protein in this complex (ILK).
As a pseudokinase, ILK is not capable to catalyze a chemical reaction, as conventional kinases do. It was therefore interesting to us why ILK still binds ATP, the small molecule normally used for catalysis, and how that relates to cell motion.”
Isabel Martin, Study First Author, Molecular Biomechanics Group, Heidelberg Institute for Theoretical Studies
Martin and her coworkers investigated the role of ATP-binding to human ILK, as well as the altered kinase dynamics and cell behavior as an outcome of ATP removal, by incorporating molecular dynamics simulations with cell biology encompassing traction force microscopy—both of which were carried out by Sara Wickström’s lab at Helsinki University.
Only by using simulations, we could analyze ILK in molecular detail, and were able to see that ATP gives structural stability to ILK. This effect is the result of an internal force propagation pathway from ATP to residues that bind an important adaptor protein. Our idea now is that ATP in ILK adopted a new and unforeseen function, namely, to aid ILK to relay mechanical forces by giving it structural stability.”
Isabel Martin, Study First Author, Molecular Biomechanics Group, Heidelberg Institute for Theoretical Studies
In a further stage, researchers confirmed the models’ predictions and proceeded beyond their time and length scales to investigate the large-scale cellular impacts of maintained ATP-binding to ILK, for which scientists worked with Finland colleagues.
Motivated by the new insights from the computer simulations done at HITS, we tested the predictions by generating cells that contained a mutated ILK protein that is not able to bind ATP. Indeed, these cells moved less efficiently than the normal cells where ATP was able to bind and strengthen ILK.”
Sara Wickström, Professor, Cell and Developmental Biology, University of Helsinki
Sara is also a faculty of the Medicine and Helsinki Institute of Life Science.
The discovery was that ATP here serves as a mechanical stabilizer—instead of its usual metabolic function—a little molecule making a huge impact. Frauke Graeter, the co-author of the paper and chief of the MBM group at HITS, outlines “Our findings add another piece in the puzzle to improve our understanding of how cells can stay where they are supposed to but move when they are required to.”
Source:
Journal reference:
Martin, I. M., et al. (2022) ATP allosterically stabilizes integrin-linked kinase for efficient force generation. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2106098119.