Following a genome-wide functional screen, scientists from the Babraham Institute’s Epigenetics research department were able to learn more about naïve stem cell reprogramming. Their study, which was published in Science Advances, identifies the key regulators of reprogramming and suggests ways to make human naïve pluripotent stem cells more efficient and quick.
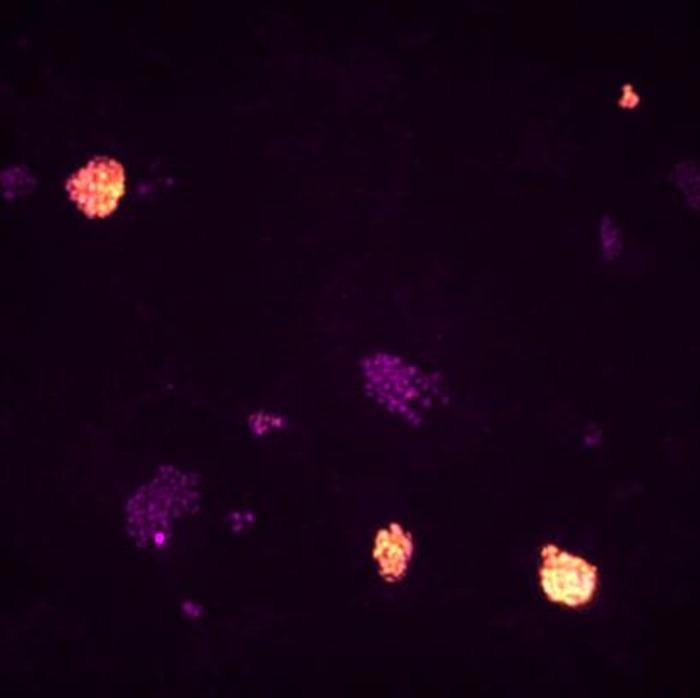 Immunofluorescent microscopy images show the different morphology of reprogrammed pluripotent stem cells (orange) and cells that were not reprogrammed (purple). Image Credit: Adam Bendall, PhD Student, the Babraham Institute.
Immunofluorescent microscopy images show the different morphology of reprogrammed pluripotent stem cells (orange) and cells that were not reprogrammed (purple). Image Credit: Adam Bendall, PhD Student, the Babraham Institute.
Human pluripotent stem cells (PSCs) are a great tool for researchers who want to learn more about how cells specialize to form each of our body’s tissues. They are available in two states: primed and naïve. Both forms of PSC have the ability to self-renew and specialize in new cell types, but their roles and molecular properties are different.
Dr Peter Rugg-Gunn stated the significance of these cells: “Human PSCs in the naïve state replicate the key molecular and cellular characteristics of cells in a pre-implantation stage embryo.”
Importantly, when naïve PSCs are encouraged to self-organize in particular conditions, they form structures that resemble an early blastocyst stage of development. By growing these cells in the lab, we can learn about the key events that happen during human development, and they have potential uses in personalized medicine. But we need to create high-quality, stable stem cell populations to be able to conduct our experiments.”
Dr Peter Rugg-Gunn, Group Leader, Epigenetics Research Program, Babraham Institute
Pluripotent stem cells are created from embryos or by removing cell identity from specialized cells utilizing Nobel Prize-winning procedures. The majority of reprogramming studies result in primed PSCs, which are further advanced in development than naïve PSCs.
Researchers can harvest naïve PSCs direct from human pre-implantation embryos, or they can expose primed PSCs to circumstances that cause them to become naïve PSCs. Existing reprogramming procedures were inefficient and slow, limiting researchers from creating large quantities of high-quality stem cells in a timely manner.
Adam Bendall, PhD student and a main researcher on the study, noted: “Very little was known about what genetic and epigenetic factors are required for naïve cell reprogramming, and this knowledge gap limited the design of reprogramming conditions.”
The low success rate of naïve reprogramming shows that there are obstacles that prevent cells from attaining the naïve state. By using a large-scale genetic screen to discover genes that impede and promote reprogramming, Adam and his colleagues were able to line up on these obstacles. Researchers were able to find a huge number of genes that play a key part in naïve PSC programming that had never been related to the process before.
The researchers focused on the PRC1.3 complex, an epigenetic complex that controls gene expression without changing the basic DNA sequence and is required for the creation of naïve PSCs. Without this complex, reprogramming cells transform into an entirely different type of cell than naïve PSCs. This shows that PRC1.3 activity might help more cells to effectively reprogramme, decreasing the barrier.
After discovering variables that support reprogramming, the researchers looked at factors that inhibit it, with an epigenetic protein called HDAC2 serving as an example in their study.
Excitingly, when we inhibited one of these factors using selective chemicals, then naïve PSC reprogramming occurred more efficiently and rapidly. We’re able to look at it from both sides; we can remove the barriers and introduce the factors that push cells towards state change.”
Dr Amanda Collier, Study First Author, Epigenetics Research Program, Babraham Institute
This study not only improves researchers’ capacity to make human naïve PSCs but also gives information on the molecular mechanisms that occur during the cell state change, some of which are preserved in human embryo developmental control.
The Rugg-Gunn team is piecing together all the pieces of a larger puzzle: the greatest knowledge of how naïve stem cells develop and are controlled. A previous study has revealed molecular components that aid in the maintenance of naïve cells.
By building up our tools for manipulating pluripotent stem cells, we can spend more time asking important questions about the pre-implantation embryo. In the longer term, further improvements in working with naïve PSCs might open up the possibility for using these cells in personalized disease models or cell therapies, although this will require more research on how to differentiate naïve PSCs into specialized cell types.”
Dr Peter Rugg-Gunn, Group Leader, Epigenetics Research Program, Babraham Institute
Source:
Journal reference:
Collier, A. J., et al. (2022) Genome-wide screening identifies Polycomb repressive complex 1.3 as an essential regulator of human naïve pluripotent cell reprogramming. Science Advances. doi.org/10.1126/sciadv.abk0013.