The reason why the control centers, or nuclei, of certain blood cancer cells, have a peculiar structure is still a mystery.
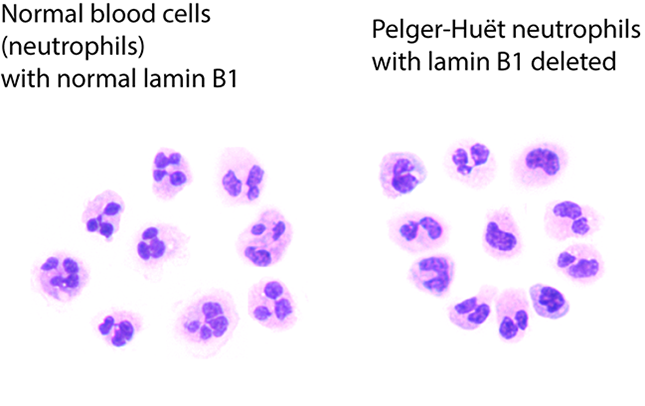 A comparison of normal neutrophils on the left, with blood cells with Pelger-Huët anomalies on the right. Image Credit: Doulatov lab
A comparison of normal neutrophils on the left, with blood cells with Pelger-Huët anomalies on the right. Image Credit: Doulatov lab
These discoveries shed light on the genesis and course of these tumors, and they may point to novel strategies to diagnose and treat specific leukemias early.
Pelger-Hut anomalies are nuclei that are compressed in the middle and resemble pince-nez spectacles. In 1928, they were first observed under a microscope. Clinical labs have long used this cellular abnormality to identify leukemias and myelodysplastic syndrome, a disorder of blood-forming cells in the bone marrow.
Although this structural shift inside blood cells suggests the possibility of cancer, no one noticed what caused it until the latest study.
Cancer biologists announce the findings of the genetic mutation that causes these cell abnormalities in the journal CELL Stem Cell.
The primary diagnosis of many cancers, even in the era of genomic medicine, remains centered on the appearance of cells under a microscope.”
Dr Sergei Doulatov, Study Senior Investigator and Associate Professor, Medicine, Division of Hematology, University of Washington School of Medicine
The study was a collaborative work among several institutions.
He said that pap smears are one type of cancer screening that looks for abnormally shaped nuclei in a patient’s cells.
Malignancies of infection-fighting white blood cells termed neutrophils are one of Doulatov’s study interests. He and his colleagues tried to figure out what was causing these malignancies at the molecular level. They reasoned that something was hidden in the DNA of the more primitive progenitor cells, or stem cells, which go on to form blood cell lineages.
What affects the fate of these progenitor cells, causing malignant cells to arise instead of normal neutrophils? Myeloid cells are progenitor cells for neutrophils, red blood cells, and platelets while they are still multipotent and capable of giving rise to any of several blood cell types.
Myeloid cells can reveal aberrant, precancerous alterations on their own. The loss of nuclear lamin B1, which is encoded on chromosome 5q, was suspected by the researchers in this latest study. When cells from improperly developing myeloid tissue are evaluated, it is typically eliminated. This study’s findings show that this loss is to blame for the nuclei’s distorted shape.
Doulatov clarified, “Lamins are proteins that line the inside of the nucleus, and are mutated in inherited disorders - famously progeria, the disorder of accelerated aging.” In malignancies, the production of lamin protein is frequently disrupted.
“We showed that loss of nuclear Lamin B1 induces defects in the nuclear morphology and human hematopoietic [blood-forming] stem cells associated with malignancy,” the researchers said.
His team went on to explain how a lack of lamin B1 affects genome organization. This resulted in the proliferation of blood-forming stem cells, a proclivity for myeloid differentiation, genomic instability due to faulty DNA damage repair, and other issues that laid the stage for cancer.
They also discovered that aberrant nuclei in the cells of myeloid pre-cancerous growths in patients were linked to deletions in the lamin1 B1 region of chromosome 5q.
According to the researchers, the deletion of lamin B1 was both essential and sufficient to generate the Pelger-Hut abnormalities. Through the organization of the genome, the researchers were also able to connect this aberrant nuclear shape to the determination of progenitor and blood-forming stem cell fate.
According to the researchers, nuclear lamin B1 is a master regulator of cell destiny specification, genomic integrity, and nuclear morphology in blood-forming stem cells.
We show that lamin B1 deletion causes changes in stem cell function, nuclear shape, and leukemia progression. Our research discovers lamin mutations in cancer and demonstrates that these mutations are responsible for the oddly shaped nuclei that have puzzled and helped pathologists recognize cancers over the past century.”
Dr Sergei Doulatov, Study Senior Investigator and Associate Professor, Medicine, Division of Hematology, University of Washington School of Medicine
The latest genetic discoveries and their implications for abnormal changes in blood-forming cells could be crucial in the treatment of leukemia in the future. According to the researchers, the existence of these alterations could be an early cancer biomarker, allowing for quicker diagnosis and treatment of leukemias.
Source:
Journal reference:
Reilly, A., et al. (2022) Lamin B1 deletion in myeloid neoplasms causes nuclear anomaly and altered hematopoietic stem cell function. Cell Stem Cell. doi.org/10.1016/j.stem.2022.02.010.