Chronic viral infections and cancers can damage the immune system permanently, diminishing the ability of immune killer T cells to destroy tumor cells or virus-infected cells—a condition known as “immune exhaustion.”
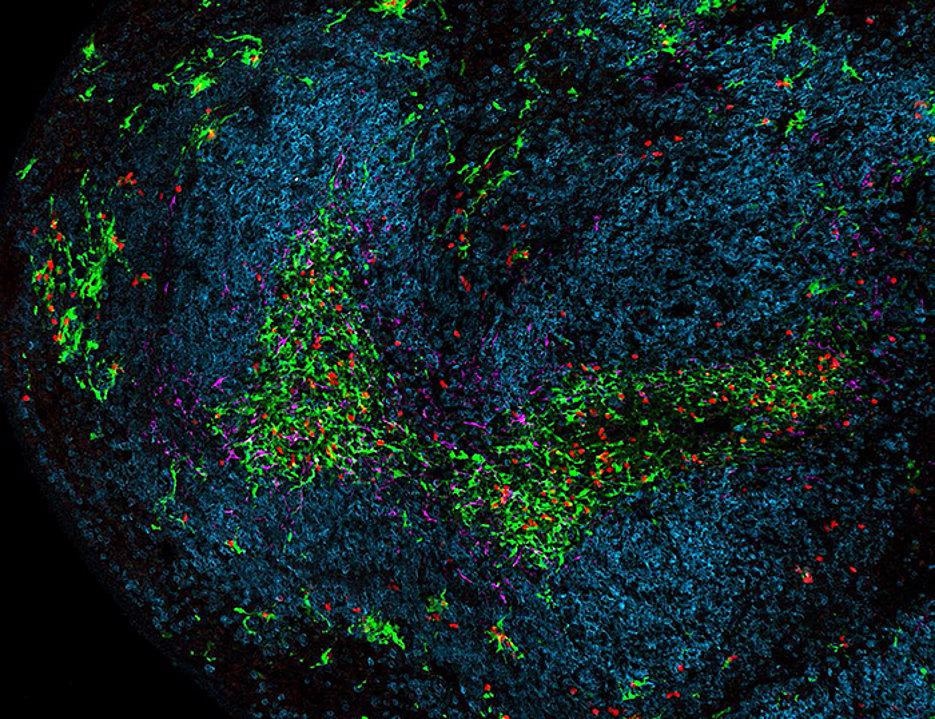 A part of the spleen during chronic viral infection. Dendritic cells are green, Killer T cells are red, B cells are blue and stromal cells are magenta. Image Credit: Team Wolfgang Kastenmüller / Universität Würzburg.
A part of the spleen during chronic viral infection. Dendritic cells are green, Killer T cells are red, B cells are blue and stromal cells are magenta. Image Credit: Team Wolfgang Kastenmüller / Universität Würzburg.
Blocking immune exhaustion using drugs like checkpoint inhibitors has shown to be a highly effective treatment for some tumors, but these immunotherapies are not always effective and can have serious adverse effects.
German-Australian cooperation
The Würzburg University-led study, which was completed in cooperation with the Peter Doherty Institute for Infection and Immunity (Doherty Institute), has developed a better understanding of how killer T cells interact with dendritic cells in the processes that lead to immune exhaustion, as well as how checkpoint inhibitors restore immune function. The results were published in the journal Immunity.
Most immune exhaustion study has focused on killer T cells, but little is known about their cellular interaction partners, according to Professor Wolfgang Kastenmüller, senior author and director of the Würzburg Institute of Systems Immunology and leader of the Max-Planck Research group, in Würzburg University.
Our results show that checkpoint immunotherapy works at the interface between killer T cells and dendritic cells. Our study used highly sophisticated microscopy to identify where these interactions between killer T cells and dendritic cells take place and how these interactions determine the outcome of a chronic viral infection following checkpoint immunotherapy.”
Professor Wolfgang Kastenmüller, Study Senior Author and Director, Würzburg Institute of Systems Immunology, Max-Planck Research Group, Julius-Maximilians-Universität Würzburg
Professor Kastenmüller stated, “We’ve shown that dendritic cells activate killer T cells just at the right level as to prevent their overactivation to avoid unwanted immunopathology. This was related to the ability of dendritic cells to maintain unique anatomical niches within lymphoid organs where killer T cells can be retained in a status ready to fight the infection at the correct moment.”
Implications for research into treatments of cancer and viral infections
Professor Sammy Bedoui, co-leader of the study and Immunology Theme Leader at the Doherty Institute in University of Melbourne, said the finding had far-reaching implications for future research into effective treatments for viral infections like AIDA and HIV, hepatitis, and possibly COVID-19, as well as specific types of skin, lung, and kidney cancers.
In the absence of dendritic cells, we have shown that checkpoint inhibitors no longer work. Instead, killer T cells got out of control, resulting in more inflammation and even poorer abilities of the immune system to control the infection.”
Professor Sammy Bedoui, Study Co-Lead Author, Department of Microbiology and Immunology, Peter Doherty Institute for Infection and Immunity, University of Melbourne
Source:
Journal reference:
Dähling, S., et al. (2022) Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity. doi.org/10.1016/j.immuni.2022.03.006.