After tumors are surgically removed, a novel biodegradable gel increases the immune system’s capacity to keep cancer away.
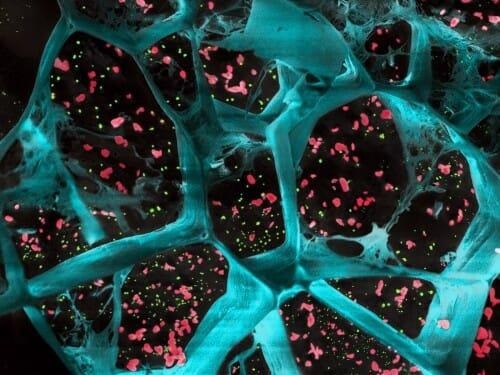 A high-magnification of the hydrogel (in blue) encapsulating T cell-activating platelets (in red) and nanoparticles that release a drug to inhibit tumor-boosting cells (in green). This gel inhibited the growth of cancer cells after surgical removal of different types of tumors. Image Credit: University of Wisconsin-Madison.
A high-magnification of the hydrogel (in blue) encapsulating T cell-activating platelets (in red) and nanoparticles that release a drug to inhibit tumor-boosting cells (in green). This gel inhibited the growth of cancer cells after surgical removal of different types of tumors. Image Credit: University of Wisconsin-Madison.
The gel, which has been tested on mice, delivers drugs and antibodies that deplete immune-blocking macrophages from the surgery site while also activating T cells to target cancer.
The gel was tested on mice models of numerous tumors by experts at the University of Wisconsin–Madison. Researchers discovered that the gel efficiently held tumors in control, including CT26 colon tumors, which are known to react well to this type of immune treatment.
The gel, on the other hand, was effective against B16F10 melanomas, S180 sarcomas, and 4T1 triple-negative breast tumors, which are less susceptible to immune therapy and more likely to spread.
These proof-of-concept studies will pave the way for more research on various animal models, which might lead to human clinical trials in the future.
The research was headed by Quanyin Hu’s group at the University of Wisconsin–Madison School of Pharmacy, with help from pharmacy professor Seungpyo Hong and collaborators at the University of Wisconsin School of Medicine and Public Health. The findings were published in the journal Nature Communications on April 6th, 2022.
We are really glad to see that this local strategy can work against so many different kinds of tumors, especially these non-immunogenic tumors. We are even more glad to see this local treatment can inhibit tumor metastasis.”
Quanyin Hu, Professor, School of Pharmacy, University of Wisconsin–Madison
Many tumors respond well to surgery, but small amounts of cancer cells left over after the procedure might cause tumors to grow again. To address this, the researchers created a gel that gently releases two critical components into the surgery site.
Pexidartinib, for example, is a drug licensed for inhibiting the activity of tumor-associated macrophages. Tumor development is aided by these cells, and blocking them reduces cancerous growth.
Platelets—blood-clotting cells—were coupled to immune-stimulating antibodies as the gel’s second component. Antibodies called anti-PD-1 aid T cells in the immune system in recognizing and attacking cancerous cells.
The researcher believes that releasing the antibody-bound platelets and Pexidartinib locally would optimize their efficacy near the tumor site while reducing the negative effects that can occur when these therapies are administered intravenously and circulate broadly throughout the body. The gel had no noticeable side effects in mice that were administered it. Over time, the gel is degraded by the body.
Because tumors respond differently to immune-based treatments like anti-PD-1-conjugated platelets, Hu’s researchers tested the gel on a variety of tumors. The gel significantly inhibited the development of lingering cancer cells and extended the lives of mice in each case. The gel also significantly slowed the spread of the researchers’ metastasizing breast cancer model.
Hong and Hu have been working on innovative approaches to managing cancers without using standard chemotherapy, which has a lot of negative effects. Researchers hope to continue trying inventive techniques that might make their way into human patients in the coming years now that researchers have teamed up.
This is just the initial phase of collaboration between our two labs.”
Seungpyo Hong, Professor, School of Pharmacy, School of Medicine and Public Health, University of Wisconsin-Madison
Source:
Journal reference:
Li, Z., et al. (2022) Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nature Communications. doi.org/10.1038/s41467-022-29388-0.