Reviewed by Danielle Ellis, B.Sc.Apr 15 2022
According to Weill Cornell Medicine researchers, inhibiting a critical signaling route in brain-resident immune cells may reduce brain inflammation and hence halt the progression of the disease in Alzheimer’s and other neurodegenerative disorders. The results indicate that new therapeutic techniques against neurodegenerative disorders, which are quite frequent in older persons and for whom there are currently no effective disease-modifying medicines.
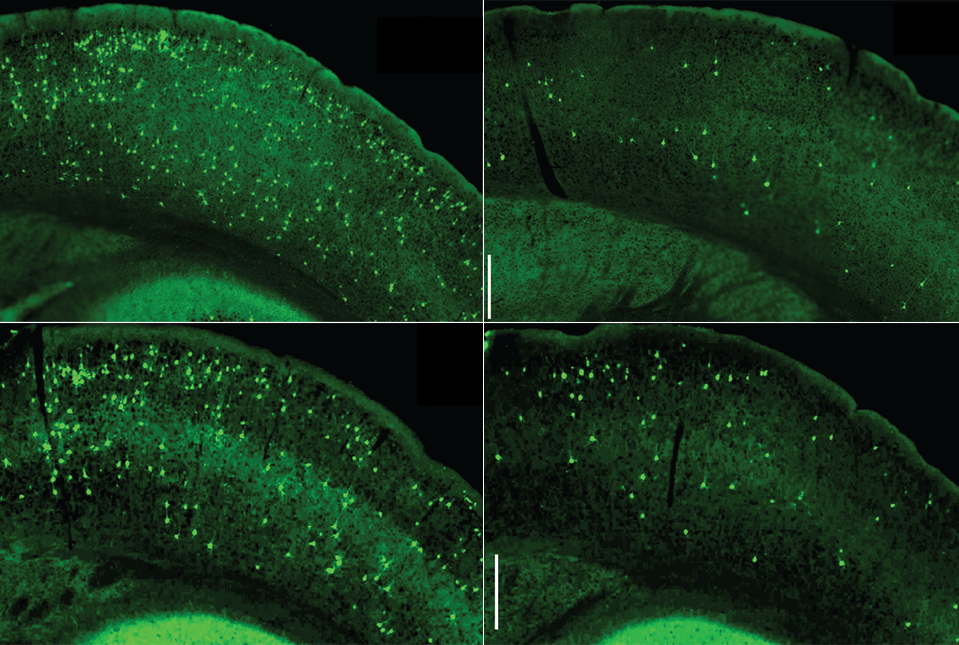 Microglial NF-κB accelerates the seeding and spread of tau aggregates in the cortex of mice inoculated with tau seeds. Depleting microglia (top right) or inhibiting microglia NF-kB (bottom right) diminished the amount of tau aggregates. (Controls are shown on the left.) Image Credit: Dr Li Gan
Microglial NF-κB accelerates the seeding and spread of tau aggregates in the cortex of mice inoculated with tau seeds. Depleting microglia (top right) or inhibiting microglia NF-kB (bottom right) diminished the amount of tau aggregates. (Controls are shown on the left.) Image Credit: Dr Li Gan
Inflammation of the brain, particularly through the activation of immune cells termed microglia, has prolonged been recognized as a prevalent characteristic of neurodegenerative disorders. Another common feature of these illnesses is the growth of aberrant, thread-like aggregates—“tangles”—of a neuronal protein called tau.
The researchers found that tau tangles assist drive inflammatory activation of microglia through a multifunctional signaling route termed the NF-κB pathway in their study. The research was published on April 12th, 2022, in the Nature Communications journal. In a tau-based Alzheimer’s mouse model, inhibiting microglial NF-κB signaling substantially pulled the immune cells out of their inflammatory state and corrected the mice’s learning and memory difficulties.
Our findings suggest restraining overactive NF-κB may be a good therapeutic strategy in Alzheimer’s and other tau-mediated neurodegenerative diseases.”
Dr Li Gan, Director, Helen and Robert Appel Alzheimer’s Disease Research Institute
Dr Li was also a Burton P. and the Judith B. Resnick Distinguished Professor in Neurodegenerative Diseases in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
In Alzheimer’s, Parkinson’s, Pick disease, progressive supranuclear palsy, frontotemporal dementia, and many other neurodegenerative illnesses, tau tangles are detected within neurons in affected brain regions.
Investigations have demonstrated that tangles can stimulate the production of additional tangles when inserted into animal brains, causing a chain reaction in which the tangles expand to other brain regions. Autopsy investigations in Alzheimer’s disease and other “tauopathies” show that the spread of tangles closely follows the progression of the disease.
The specific involvement of tangles in brain cell death has never been determined. Prior research has revealed that tau tangles engage with microglia in a way that causes them to become inflammatory and disease-associated.
The microglia, which ordinarily try to eat the tau tangles, become ineffective in this inflammatory state. Much of the tau is cleared away from the microglia in forms that prefer to establish new tangles rather than being digested.
Dr Gan and her colleagues discovered evidence in cell culture and mouse tests that tau tangles push microglia into this disease-linked inflammatory state by triggering the NF-κB signaling pathway.
They demonstrated that keeping the NF-κB pathway hyperactive in microglia improved the seeding and propagation of tangles, which promote additional NF-κB activation, in an Alzheimer’s mouse model with tau-tangles mostly driven by seeded tau. Shutting down NF-κB, on the other hand, stopped the vicious loop and significantly reduced the spread of the tangles.
The researchers demonstrated that inactivating microglial NF-κB switched the microglia nearly completely out of their inflammatory, disease-associated condition, regaining a much more normal cell structure and gene activity pattern in some other tau animal models with tau tangles produced in aged neurons.
This change, which prevents microglia from disgorging harmful tau seeds, protected the mice from developing important cognitive and memory problems in this scenario.
“Taken together, our experiments suggest that tau’s toxic effects on cognition require microglial NF-κB signaling,” said co-senior author Dr Wenjie Luo, associate professor of research in neuroscience in the Appel Alzheimer’s Disease Research Institute and the Feil Family Brain and Mind Research Institute at Weill Cornell.
Many experimental Alzheimer’s therapies have tried to slow or stop the progression of the disease by attacking amyloid plaques and, more recently, tau tangles over the last two decades. All of these attempts have so far underperformed in large-scale clinical studies. According to Dr Gan, the latest discoveries suggest that future treatments targeting hyperactive microglial NF-κB signaling may have a better chance of success.
Her lab is now conducting an additional study to learn more about how microglial NF-κB signaling, which impacts the activity of hundreds of other microglial genes, damages neurons and causes cognitive and memory problems. The researchers will look into how to control certain components of hyperactive NF-κB signaling without compromising the immune cells’ normal function in the brain.
Source:
Journal reference:
Wang, C., et al. (2022) Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nature Communications. doi.org/10.1038/s41467-022-29552-6.