Reviewed by Danielle Ellis, B.Sc.Apr 18 2022
Professor Qiuyu Zhang (Northwestern Polytechnical University), Professor Ki-Bum Lee (Rutgers University), and Professor Liang Kong (School of Stomatology, The Fourth Military Medical University) directed this research. They developed an injectable hybrid inorganic (IHI) nanoscaffold-templated stem cell assembly that they used to regenerate critical-sized cartilage lesions.
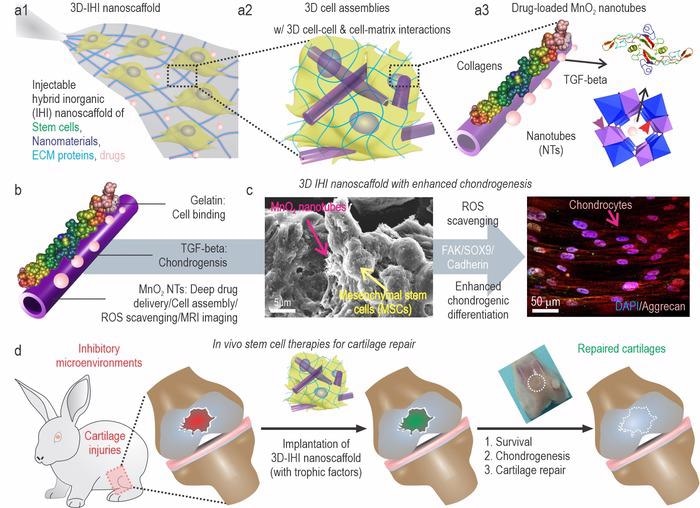 a) A schematic illustration of 3D TGFβ-BMSC-IHI nano scaffold. b) The schematic illustration of gelatin-coated and TGF-β3-loaded MnO2 NTs. c) The FESEM image indicated that most of the BMSCs form contacts with other cells and the 1D fibril-like structures, which was similar to the structures of natural tissues. d) By remodeling the oxidative microenvironment, enhancing cell viability, and chondrogenesis of transplanted cells, cartilage regeneration could be finally achieved. Image Credit: ©Science China Press
a) A schematic illustration of 3D TGFβ-BMSC-IHI nano scaffold. b) The schematic illustration of gelatin-coated and TGF-β3-loaded MnO2 NTs. c) The FESEM image indicated that most of the BMSCs form contacts with other cells and the 1D fibril-like structures, which was similar to the structures of natural tissues. d) By remodeling the oxidative microenvironment, enhancing cell viability, and chondrogenesis of transplanted cells, cartilage regeneration could be finally achieved. Image Credit: ©Science China Press
Cartilage injuries are generally fatal, and most of them are incurable due to cartilage tissues’ innately limited regeneration ability. Developmental biology, disease modeling, and regenerative medicine have all benefited from the introduction of 3D stem cell cultivation platforms.
For instance, once effectively transplanted, stem cells could secrete trophic factors to reduce inflammation at cartilage injury sites before differentiating into cartilage cells (e.g., chondrocytes) to restore function.
Despite this, many obstacles must be overcome before stem cell therapies’ therapeutic potential may be achieved. The lack of control over stem cell chondrogenic differentiation in vivo has frequently resulted in poor restorative outcomes. Furthermore, stem cells typically undergo apoptosis after injection due to the presence of oxidative stress and inflammation in the microenvironment of damaged sites.
Image Credit: Crevis/Shutterstock.com
They exhibited the creation of a 3D IHI nanoscaffold-templated stem cell assembly technology for enhanced 3D stem cell culture and implantation to solve these problems.
Through customized 3D cell-cell and cell-matrix interactions, the 3D-IHI nanoscaffold quickly arranges stem cells into injectable tissue constructs, delivers chondrogenic proteins intensely and uniformly in the assembled 3D culture systems, and controllably provokes chondrogenesis via nanotopographical effects.
The 3D-IHI nanoscaffold effectively governs the dynamic microenvironment after cartilage injury by integrating the aforementioned regenerative cues and concurrently scavenging reactive oxygen species utilizing manganese dioxide-based proportion once embedded in vivo in a rabbit cartilage injury model.
In this method, both short- and long-term healing of cartilage abnormalities, as well as fast tissue regeneration and functional recovery, can be achieved. Because of its adaptability and therapeutic results, 3D-IHI nanoscaffold-based cartilage regeneration could be a potential way to develop several tissue engineering applications.
Source:
Journal reference:
Wang, S., et al. (2022) Injectable stem cell assembly for cartilage regeneration. National Science Review. doi.org/10.1093/nsr/nwac037.