It is highly regulated what goes into the brain and what does not. The phagocytes that encapsulate the blood arteries in the brain and maintain the blood-brain barrier have been examined by researchers at the University of Freiburg’s Faculty of Medicine.
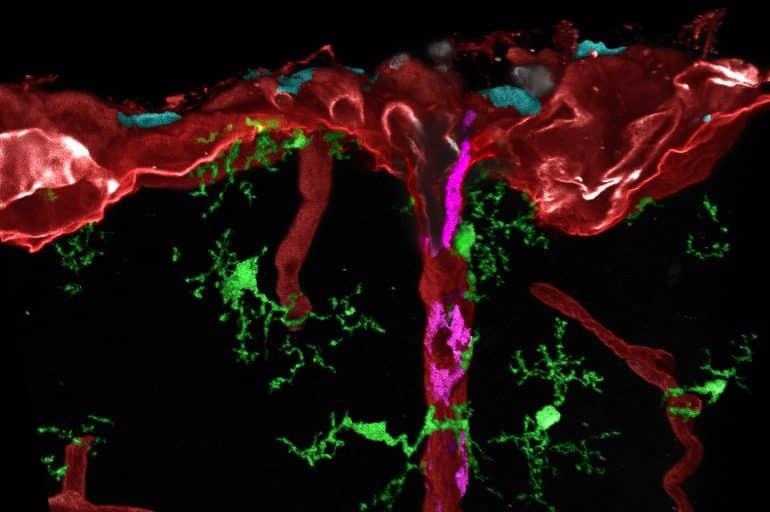 Post-processed microscopic image of different tissue macrophages (cyan, magenta or green) in mouse brain. Image Credit: Medical Center–University of Freiburg / Dr Lukas Amann.
Post-processed microscopic image of different tissue macrophages (cyan, magenta or green) in mouse brain. Image Credit: Medical Center–University of Freiburg / Dr Lukas Amann.
These cells only grow fully after birth, according to a set step-by-step developmental pattern, as experts from the Institute of Neuropathology at the Medical Center–University of Freiburg and an international research group have demonstrated. This procedure was previously thought to be finished during embryonic development.
Their research, which was published in the journal Nature on April 20th, 2022, began with genetically engineered mouse lines and was later corroborated using human samples. Researchers are anticipated to yield crucial information.
We were able to show that the immune cells we studied migrate from the cerebral membrane to the blood vessels in the brain shortly before birth and mature there. This process is probably not completed until weeks after birth and could partly explain why the brain is so vulnerable at the beginning of life. The late timing of the maturation of the phagocytes, also called macrophages, was very surprising to us, since the precursor cells are already present in the brain long before.”
Dr Marco Prinz, Professor, Medical Director of the Institute of Neuropathology, Medical Center–University of Freiburg
Prinz is also a director of the University of Freiburg’s Collaborative Research Center/Transregio 167–NeuroMac and a participant of the CIBSS–Centre for Integrative Biological Signaling Studies Cluster of Excellence.
Furthermore, the researchers were able to demonstrate for the very first time that the vessels, and structure-giving cells in the brain, provide critical signals for correct macrophage growth.
The blood-brain barrier is formed by cells that line the brain’s blood arteries. They regulate which chemicals are allowed to enter the brain and which are not. This shields the brain from infections and toxic chemicals. Infectious diseases, some brain tumors, and oxygen shortage all cause the blood-brain barrier to break down.
Significance for Alzheimer’s, multiple sclerosis and more
In addition to the blood-brain barrier, the immune cells we studied control what can reach the brain cells from the blood, they eat pathogens and prevent excessive inflammation. They are also involved in the development of cancer, Alzheimer's disease and multiple sclerosis. Our findings could be important for a better understanding of these diseases and future therapies.”
Dr Marco Prinz, Professor, Medical Director of the Institute of Neuropathology, Medical Center–University of Freiburg
Color-coded cells and gene analyses Farbmarkierte Zellen und Gen-Analysen
The researchers, headed by the two first authors, Dr Takahiro Masuda of Kyushu University in Japan and Dr Lukas Amann of the University of Freiburg’s Faculty of Medicine, employed multiple newly developed mouse lines for their study.
Different kinds of brain macrophages and their progenitor cells may be tagged for the first time with these, and then discovered in different brain areas using high-resolution microscopy. Furthermore, they examined the gene activity of individual cells to evaluate their maturity level.
We were also able to confirm the data on human brains. This gives us a much deeper understanding of the timing and molecular mechanisms in the development of the cells. This knowledge can now be used to explore new and more specific therapeutic approaches for brain diseases.”
Dr Lukas Amann, Biologist, Faculty of Medicine in the Institute of Neuropathology, Medical Center–University of Freiburg
The study included scientists from Freiburg, Berlin, Hannover, Leipzig, Japan, Sweden, France, and the United States.
Source:
Journal reference:
Masuda, T., et al. (2022) Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature. doi.org/10.1038/s41586-022-04596-2.