Glioblastomas (GBMs) are cancerous tumors of the brain and spinal cord that are incredibly aggressive. Brain cancers, such as GBM, are difficult to treat since many cancer therapeutic approaches cannot cross the blood-brain barrier, and more than 90% of GBM tumors recur after surgery, despite surgery and successive chemo and radiation therapy being the most effective way of treating the disease.
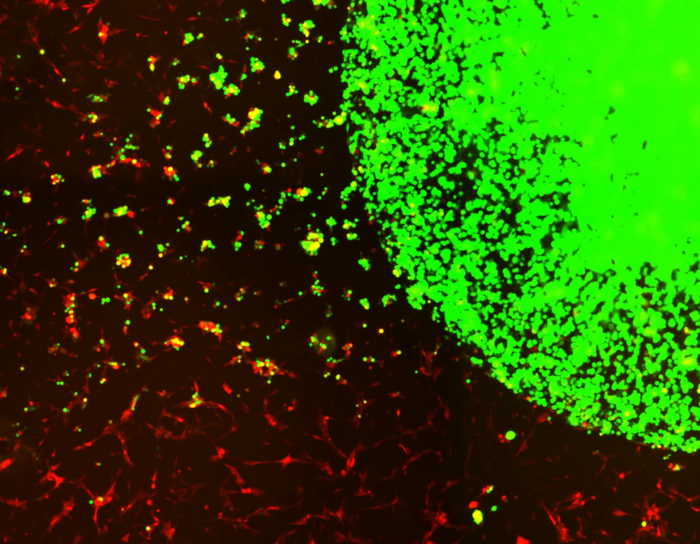 Encapsulated stem cells (green) tracking and killing GBM tumor cells (red). Image Credit: Shah lab (CSTI)
Encapsulated stem cells (green) tracking and killing GBM tumor cells (red). Image Credit: Shah lab (CSTI)
In a new study conducted by researchers at Brigham and Women’s Hospital and Harvard Medical School, the a novel treatment approach is developed for curing GBMs after surgery by using stem cells derived from healthy donors that have been engineered to attack GBM-specific tumor cells.
This strategy displayed impressive effectiveness in preclinical studies of GBM, with 100% of mice living for more than 90 days after treatment. The findings were published in Nature Communications.
This is the first study to our knowledge that identifies target receptors on tumor cells before initiating therapy, and using biodegradable, gel-encapsulated, ‘off-the-shelf’ engineered stem cell-based therapy after GBM tumor surgery.”
Khalid Shah, MS, PhD, Director, Center for Stem Cell and Translational Immunotherapy, Brigham and Women’s Hospital
Shah was also the vice-chair of research in the Department of Neurosurgery at the Brigham and faculty at Harvard Medical School and Harvard Stem Cell Institute (HSCI).
“In the future, we will be applying this strategy to promptly identify target receptors after one receives a GBM diagnosis, then administer a gel-encapsulated, off-the-shelf, engineered stem cell therapy from a pre-made reservoir,” Shah added.
Numerous cancer cell-based therapies are obtained from the patient’s stem cells or immune cells. Moreover, due to the disease’s rapid evolution, most patients with GBM undergo surgery within the first week of being diagnosed, leaving little time to build therapeutics from their cell types.
Instead, researchers devised a novel method of using allogeneic stem cells, or cells from healthy people, to ensure that the remedy is easily accessible to administer at the time of surgery.
Shah and colleagues investigated the efficacy of various capsules used to transport stem-cell therapies into the brain and discovered that a biodegradable hydrogel capsule successfully transported the treatment without being swept away by cerebrospinal fluid.
Using a genetic biomarker commonly expressed on tumor cells, scientists first recognized “death receptors” on circulating tumor cells (CTC), or cancer cells in the bloodstream.
They isolated stem cells from the bone marrow of proper human donors and engineered them to discharge a protein that unites death receptors and causes cell death.
Researchers also constructed a safety mechanism into the stem cell system which allows PET imaging to track stem cells and, when powered up, eliminates stem cells and increases cancer cell death. Eventually, Shah’s team evaluated the effectiveness of the therapeutic bifunctional cells (MSCBif) in post-surgery animal models of primary and recurrent GBM tumors.
Prominently, all mice that gained the gel-encapsulated stem cell-based therapeutic after surgery survived for 90 days, as contrasted to mice that only received surgery, which had an average survival time of 55 days. Researchers also tested the safety of this clinical treatment on mice in several studies using different doses of MSC therapy. Researchers discovered no toxicity in mice with or without tumors.
The findings of this study pave the way for phase I clinical trials in patients with GBM undergoing brain surgery over the next two years. According to Shah and his peers, this treatment approach will be relevant to a broader range of solid tumors, and further research into its applications is guaranteed.
Beyond this therapy’s significant exhibited success rate, these findings suggest that we can use stem cells from healthy individuals to treat cancer patients. This work lays down a foundation to begin building an engineered therapeutic stem cell biobank targeting different receptors on tumor cells and the immune cells in the tumor microenvironment that we will one day be able to use to treat a wide range of difficult-to-treat cancers like GBM.”
Khalid Shah, MS, PhD, Director, Center for Stem Cell and Translational Immunotherapy, Brigham and Women’s Hospital
Source:
Journal reference:
Bhere, D., et al. (2022) Target receptor identification and subsequent treatment of resected brain tumors with encapsulated and engineered allogeneic stem cells. Nature Communications. doi.org/10.1038/s41467-022-30558-3.