Research studying the autoimmune response, in which the immune system kills the insulin-producing pancreatic islet beta cells, is a common topic of type 1 diabetes research. However, a recent investigation by researchers at the University of Chicago examines how beta cells themselves contribute to the development of autoimmunity.
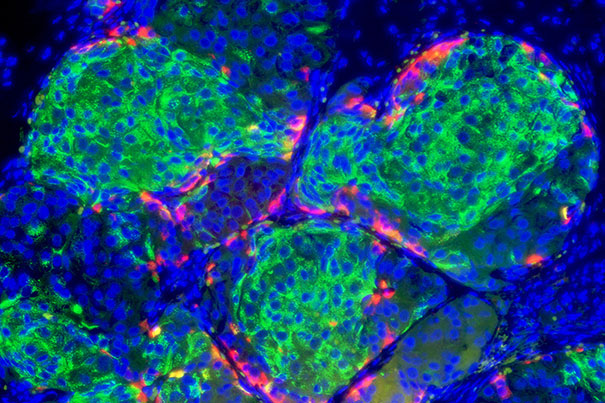 Insulin-producing pancreatic beta cells (green) derived from human embryonic stem cells that have formed islet-like clusters in a mouse. The red cells are producing another metabolic hormone, glucagon, that regulates blood glucose levels. Blue indicates cell nuclei. Image Credit: Photo by B. D. Colen/Harvard Staff; Image courtesy of Doug Melton
Insulin-producing pancreatic beta cells (green) derived from human embryonic stem cells that have formed islet-like clusters in a mouse. The red cells are producing another metabolic hormone, glucagon, that regulates blood glucose levels. Blue indicates cell nuclei. Image Credit: Photo by B. D. Colen/Harvard Staff; Image courtesy of Doug Melton
The study also suggests that new drugs might stop the immune system from damaging beta cells and stop the establishment of type 1 diabetes in susceptible or early-onset patients.
The research, which was just released in Cell Reports, details how the researchers utilized genetic methods to remove or wipe out the Alox15 gene in mice with a genetic predisposition to type 1 diabetes. An enzyme called 12/15-Lipoxygenase, which is produced by this gene, is known to engage in mechanisms that cause inflammation in beta cells.
In these animals, deleting Alox15 conserved the number of beta cells, decreased the number of immune T cells entering the islet milieu, and stopped the development of type 1 diabetes in both males and females. Additionally, these mice displayed increased expression of the gene that produces the PD-L1 protein, which inhibits autoimmunity.
The immune system doesn’t just decide one day that it’s going to attack your beta cells. Our thinking was that the beta cell itself has somehow fundamentally altered itself to invite that immunity.”
Raghavendra Mirmira, MD, PhD, Study Senior Author and Professor, Medicine, University of Chicago
Mirmira was also the Director of the Diabetes Translational Research Center.
“When we got rid of this gene, the beta cells no longer signaled to the immune system and the immune onslaught was completely suppressed, even though we didn’t touch the immune system,” he said. “That tells us that there is a complex dialogue between beta cells and immune cells, and if you intervene in that dialogue, you can prevent diabetes.”
The research is the outcome of a protracted partnership that started when Mirmira and many lab members were students at Indiana University.
Jerry Nadler, MD, Dean of the School of Medicine, found the 12/15-Lipoxygenase enzyme’s function, and Professor of Medicine and Pharmacology at New York Medical College and Maureen Gannon, PhD, Professor of Medicine, Cell and Developmental Biology, Molecular Physiology, and Biophysics at Vanderbilt University provided the study’s mice.
This strain of mice permitted the knockout of the Alox15 gene when offered the drug tamoxifen.
Research Associate Professor at the University of Chicago and co-senior author of the latest research Sarah Tersey, PhD, oversaw a project in 2012 that was among the first to propose that the beta cell might play a key role in the onset of type 1 diabetes.
“This allows us to understand the underlying mechanisms leading to the development of type 1 diabetes,” Tersey said. “This has been a huge, changing part of the field where we focus more on the role of beta cells and not just autoimmunity.”
Interesting linkages between the findings and cancer therapies that use the immune system to combat tumors are also present.
To weaken the immune system and get around the body’s defenses, cancer cells frequently express the PD-L1 protein. Inhibiting or disabling the PD-L1 “checkpoint” with new medications known as checkpoint inhibitors frees the immune system to assault malignancies.
Elevated PD-L1 in the knockout mice in the new research achieves its desired result by blocking the immune system from attacking the beta cells, as shown by the results.
In the latest research, human beta cells were also subjected to testing with a medication that suppresses the 12/15-Lipoxygenase enzyme. They discovered that the medication, ML355, raises PD-L1 levels, indicating that it might stop the autoimmune response and stop the onset of diabetes.
It should ideally be administered to people who are at high risk due to a family history of the disease and exhibit early indicators of type 1 diabetes, or soon after diagnosis before the pancreas has sustained too much damage. Clinical trials to assess a potential treatment employing ML355 are currently being initiated by Mirmira and his colleagues.
This study certainly suggests that inhibiting the enzyme in humans can increase levels of PD-L1, which is very promising. With beta cell-targeted therapeutics, we believe that as long as the disease hasn’t progressed to the point that there’s massive destruction of beta cells, you can catch an individual before that process starts and prevent the disease progression altogether.”
Raghavendra Mirmira, MD, PhD, Study Senior Author and Professor, Medicine, University of Chicago
Source:
Journal reference:
Piñeros, A. R., et al. (2022) Proinflammatory signaling in islet β cells propagates invasion of pathogenic immune cells in autoimmune diabetes. Cell Reports. doi.org/10.1016/j.celrep.2022.111011.