University of Cape Town (UCT) researchers have used cryo-electron microscopy to identify the first complete structures of the human angiotensin-converting enzyme (ACE) (cryo-EM). A protein that controls blood pressure, ACE, is essential for maintaining good heart health.
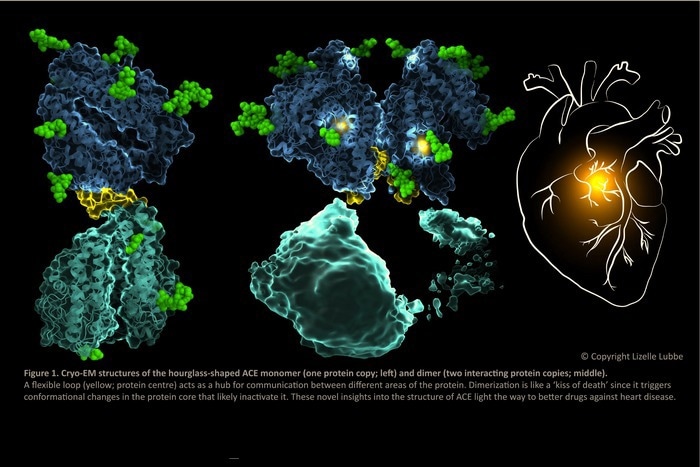 Cryo-EM structures of the hourglass-shaped ACE monomer (one protein copy; left) and dimer (two interacting protein copies; middle). A flexible loop (yellow; protein center) acts as a hub for communication between different areas of the protein. Dimerization is like a ‘kiss of death’ since it triggers conformational changes in the protein core that likely inactivate it. These novel insights into the structure of ACE light the way to better drugs against heart disease. Image Credit: Lizelle Lubbe.
Cryo-EM structures of the hourglass-shaped ACE monomer (one protein copy; left) and dimer (two interacting protein copies; middle). A flexible loop (yellow; protein center) acts as a hub for communication between different areas of the protein. Dimerization is like a ‘kiss of death’ since it triggers conformational changes in the protein core that likely inactivate it. These novel insights into the structure of ACE light the way to better drugs against heart disease. Image Credit: Lizelle Lubbe.
The cryo-EM structures of ACE in two distinct conformations, which were published on July 12th, 2022, in The EMBO Journal, have the potential to enhance treatment development for cardiovascular disease, which is the leading cause of mortality in the world.
The study was a component of START (Synchrotron Techniques for African Research and Technology), a £3.7 million initiative supported by a Global Challenges Research Fund grant from the Science and Technology Facilities Council of the UK Research and Innovation (STFC).
The research was carried out by Dr Lizelle Lubbe, Dr Jeremy Woodward, Professor Ed Sturrock, and Professor Trevor Sewell. The Sturrock Laboratory at UCT synthesized the ACE protein, and the Electron Microscope Unit at UCT processed it for high-resolution imaging (EMU).
It was moved to the UK’s national synchrotron, Diamond Light Source (Diamond), for high-resolution imaging at the electron Bio-Imaging Centre (eBIC). Both the EMU and the CSIR Centre for High-Performance Computing (CHPC) in South Africa were used for image processing.
Since it generates the hormone Angiotensin II, which constricts blood vessels and increases blood pressure, ACE is a major target for the treatment of hypertension (elevated blood pressure) as well as cardiovascular disease. Heart failure, heart attacks, renal disease, strokes, and eyesight loss are all greatly increased by hypertension. It is often asymptomatic and is referred to as “the silent killer.”
The monomeric version of ACE (one copy of the protein) is noteworthy since it consists of two connected domains that are structurally identical but functionally different. Additionally, it may be found in the study’s observed functionally significant dimeric form, which consists of two interacting copies of the protein.
The function of ACE and its drug-binding characteristics, which are essential for therapeutic drug design, is influenced by communication between the various sections of ACE.
Clinically, ACE inhibitors are recommended as one of the first-line treatments for hypertension, but they non-selectively target both ACE domains and thereby trigger side effects in some patients. It is really important to understand the structure and dynamics of these newly seen forms of ACE because this could help identify novel sites for the design of domain-selective inhibitors that avoid such side effects.”
Professor Edward Sturrock, Study Principal Investigator, Department of Integrative Biomedical Sciences, Institute of Infectious Disease and Molecular Medicine, University of Cape Town
The results of the research provide a novel insight into the extremely dynamic nature of ACE and the processes behind dimerization and communication across its many domains.
By changing from an active-site centered to a holistic view of this vital protein, we obtained valuable new insights into how ACE works.”
Dr Lizelle Lubbe, Study First Author, Department of Integrative Biomedical Sciences, Institute of Infectious Disease and Molecular Medicine, University of Cape Town
Study co-author Professor Sewell adds, “The dynamic nature of ACE prevents crystal formation, which meant that X-ray crystallography studies over the past two decades could only solve parts of the structure. We discovered many clues using this method but were unable to solve the full puzzle until we had access to high-resolution cryo-EM.”
The protein was quickly chilled to −180 °C to acquire the entire structure, trapping the various conformations in a very thin, glass-like water layer at the EMU. After that, imaging was done at eBIC using a cutting-edge Titan Krios microscope.
Even with high-resolution imaging, the unique shape, small size, and dynamic nature of ACE posed many challenges.”
Dr Jeremy D Woodward, Electron Microscope Unit, University of Cape Town
Dr Lubbe states, “Recently developed cryo-EM image processing methods were crucial to solving the structures. We had to separate the images computationally through extensive classification, amounting to ‘digital purification’ because biochemical methods failed to separate the monomeric and dimeric forms of ACE. We could then solve both ACE structures by focusing the 3D refinement on different parts of the structure in turn.”
“We are delighted with the findings of this study achieved by a brilliant team of scientists in Africa, using eBIC’s advanced cryo-EM at Diamond,” says Professor Chris Nicklin the project’s principal investigator and the leader of the Diamond Science Group under the GCRF START award.
“This is an excellent example of UK and African research partnerships and global impact through the very successful GCRF START grant. The world urgently needs sustainable solutions for killer heart diseases and other chronic health conditions. We are very excited that the study’s structural insights could pave the way for improved antihypertensive drug design,” Professor Nicklin concludes.
Source:
Journal reference:
Lubbe, L., et al. (2022) Cryo-EM reveals mechanisms of angiotensin I-converting enzyme allostery and dimerization. The EMBO Journal. doi.org/10.15252/embj.2021110550.