In a first-of-its-kind blueprint, scientists at the UCLA Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research have shown how stem cells mature into sensory interneurons, which are responsible for feelings including touch, pain, and itching.
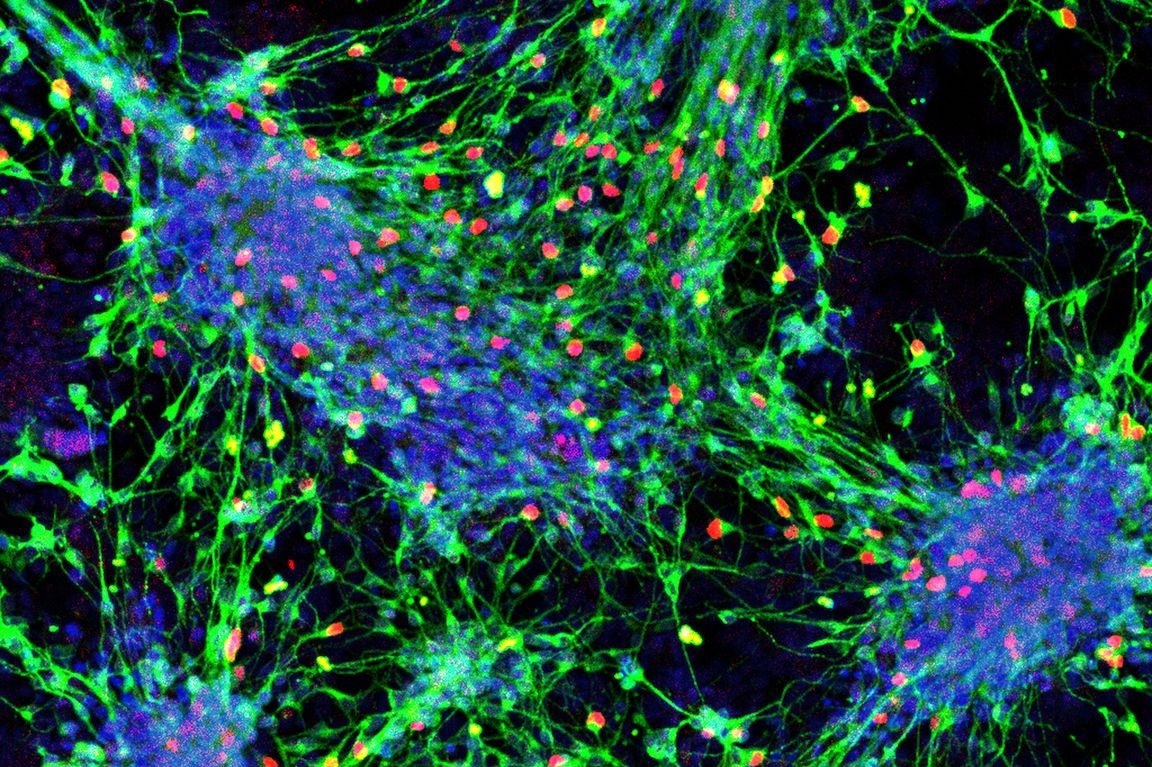 Sensory interneurons (with nuclei in red) derived from mouse stem cells. Image Credit: UCLA Broad Stem Cell Research Center.
Sensory interneurons (with nuclei in red) derived from mouse stem cells. Image Credit: UCLA Broad Stem Cell Research Center.
The scientists also discovered a technique for creating all varieties of sensory interneurons in the lab using mouse embryonic stem cells. According to the researchers, if this study can be repeated using human stem cells, it might be a significant step toward the creation of stem cell-based treatments that restore sensation in patients who have lost feeling in certain portions of their bodies owing to spinal cord injury.
For many years, my lab’s research has focused on helping people who have experienced spinal cord injuries feel again. This work brings us closer to that goal by outlining the recipe for producing the cells people need to experience the comfort of a hug or sense danger through pain.”
Samantha Butler, Study Senior Author and Professor, Department of Neurobiology, David Geffen School of Medicine, University of California–Los Angeles Health Sciences
Butler is also a Member of the Broad Stem Cell Research Center.
Recently, the research results were published in the peer-reviewed journal Cell Reports.
A group of neurons in the spinal cord called sensory interneurons are in charge of transmitting sensory data from different parts of the body to the brain. The first team to develop sensory interneurons from human embryonic and induced pluripotent stem cells was Butler’s lab in 2018. Only a small subset of these crucial cell subtypes could be produced at the time according to their findings.
By providing comprehensive methods that can be utilized to instruct stem cells to develop into each of the six sensory interneuron subtypes, this new publication builds on earlier research. The researchers have reason to expect the interneurons produced using these techniques will have the same sensory functions since they are genetically and molecularly identical to their real-life counterparts in the body.
Each subtype of sensory interneuron transmits information about a different sensation, such as touch, pressure, stretch, pain, itch and heat. Any cell therapy used to treat spinal cord injuries and restore sensation will need to contain all of these subtypes, and the subtypes need to resemble real interneurons as closely as possible.”
Sandeep Gupta, Study First Author and Postdoctoral Fellow, Department of Neurobiology, University of California–Los Angeles Health Sciences
Additionally, Gupta and Butler were able to separate pure populations of each of the six subtypes of sensory interneurons, whether they were produced from stem cells or taken directly from the body. Researchers did this by identifying distinctive markers on the cell surfaces of each of the six subtypes. In the final stage, scientists showed that by altering these methods, they could effectively produce the significant numbers of sensory interneurons required for therapeutic applications.
When combined, these developments may be very helpful for discovering novel drugs. To test medications meant to limit their activity in persons with chronic pain, researchers may, for instance, separate the sensory interneurons that convey pain signals.
This opens up the possibility that we could recreate the neural circuits that regulate pain in the lab and then screen for new drugs that function like morphine but don’t target the brain, which could reduce the risk of addiction.”
Samantha Butler, Study Senior Author and Professor, Department of Neurobiology, David Geffen School of Medicine, University of California–Los Angeles Health Sciences
Butler leads the Eleanor I. Leslie Chair in Pioneering Brain Research in the field of neurobiology.
The ability to create sensory interneurons in the lab may have implications for the development of cell therapies and drug screening in addition to allowing researchers to model, research, and ultimately find treatments for conditions that affect sensation, such as autism spectrum disorders or chronic pain sensitivity.
The group is now attempting to use human cells to confirm these results. Butler noted that if this research is successful in identifying every human sensory interneuron subtype, it would open up a vital new research path for the study of sensory processing disorders.
Source:
Journal reference:
Gupta, S., et al. (2022) In vitro atlas of dorsal spinal interneurons reveals Wnt signaling as a critical regulator of progenitor expansion. Cell Reports. doi.org/10.1016/j.celrep.2022.111119.