A minimally invasive technique was created and improved by scientists at Terasaki Institute for Biomedical Innovation (TIBI) to develop immunotherapeutic cancer treatments. These treatments are more effective, sustained, and targeted. Such a focused strategy reduces the higher dosages and potentially harmful side effects that are associated with more systemic therapy approaches.
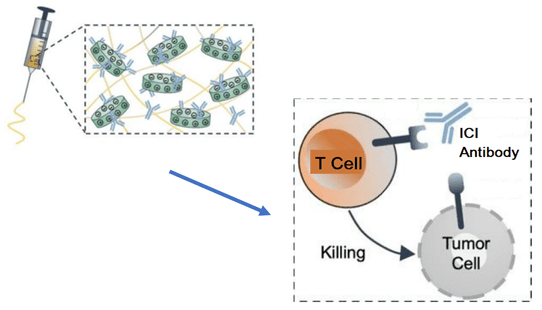 Researchers have developed a method for injectable, targeted delivery of immunotherapeutic treatments. The shear-thinning biomaterial is loaded with the ICI, which can then be delivered and released at the tumor site. The ICIs bind to T cells, preventing binding and T cell suppression by the tumor cells. This frees the T cells to seek and attack the tumor cells. Image Credit: Terasaki Institute
Researchers have developed a method for injectable, targeted delivery of immunotherapeutic treatments. The shear-thinning biomaterial is loaded with the ICI, which can then be delivered and released at the tumor site. The ICIs bind to T cells, preventing binding and T cell suppression by the tumor cells. This frees the T cells to seek and attack the tumor cells. Image Credit: Terasaki Institute
The body can react to aberrant cells or foreign invaders in a variety of ways. Immune system T-cells, which have “checkpoint proteins” on their exterior, play a role in one method. The activity of T-cells can be stimulated or suppressed because these checkpoint proteins bind to proteins on the surface of other cells.
T-cell stimulation results in the eradication of abnormal or invasive cells, whereas T-cell suppression serves as a built-in safeguard against the immune system’s attack on the body’s healthy cells.
However, tumor cells occasionally have surface proteins that trick the immune system by attaching to T-cells and inhibiting their function, allowing the tumor cells to proliferate and spread. “Immune checkpoint inhibitor” antibodies (ICIs), which prevent tumor cell attachment to T cells, have been produced in recent years.
As a result, the immunological response of the T-cell to eliminate tumor cells is reactivated. ICIs have been used successfully in the US to treat malignancies of the head and neck, kidney, bladder, and liver.
Although these antibodies have shown efficacy, they are frequently provided by systemic injection, and their effects differ from patient to patient. The non-specific nature of this administration may cause hazardous adverse effects in some patients due to excessive T-cell activation. The efficiency of the ICI is further diminished by systemic drug administration, necessitating more expensive dosages.
The key component of the TIBI team’s strategy was an injectable gelatin biomaterial with silicate nanoplatelets in the shape of discs mixed in. When delivering the ICI-loaded biomaterial to the tumor location through minimally invasive injection, the nanoplatelets’ charged surfaces were ideal for protective binding to ICIs.
The combination of gelatin and nanoplatelets was enhanced for more efficient ICI delivery and prolonged drug release. Additionally, the group showed that a variety of variables, including nanoplatelet, gelatin, ICI concentration, pH, and environments for biomaterial degradation, could be adjusted to regulate ICI released in a manner that is optimal for particular tumors.
To assess the effectiveness of their shear-thinning biomaterial (STB), which flexes under stress during injection and rebounds swiftly afterward, the researchers carried out further tests. ICIs were injected into mouse melanoma tumors using these STBs.
These effects occurred largely and were maintained over a prolonged duration than the bad control samples, which is attributable to the consistent ICI release and delivery.
They also showed that melanoma tumors in these mice showed the slowest tumor growth and smallest size, as well as straightforward margins between the tumor and skin layer and an absence of inflammation and necrotic tissue.
The samples with STB-delivered ICIs contained almost 44% more T helper cells and almost 36% more T killer cells than those of the negative controls when the number of T cells activated because ICI delivery was counted.
The demise of tumor cells was examined. Studies using various staining methods revealed that tumor cell death in STB-delivered samples could be up to 13.2 times more than in negative control samples.
The results obtained here clearly demonstrate the effectiveness of targeted, controllable and sustainable antibody delivery to reinstate the body’s natural defense mechanisms against cancer.”
Ali Khademhosseini, PhD, Director and CEO, Terasaki Institute for Biomedical Innovation
“Its potential in creating combination therapies further extends its impact,” he added.
Source:
Journal reference:
Wu, Q., et al. (2022) A Shear-Thinning Biomaterial-Mediated Immune Checkpoint Blockade. ACS Applied Materials & Interfaces. doi.org/10.1021/acsami.2c06137.