Researchers from UC, the University of Illinois Urbana-Champaign, and the University at Buffalo demonstrated that light-activated proteins can help normalize dysfunction within cells. The study was published in the journal Nature Communications on July 25th, 2022.
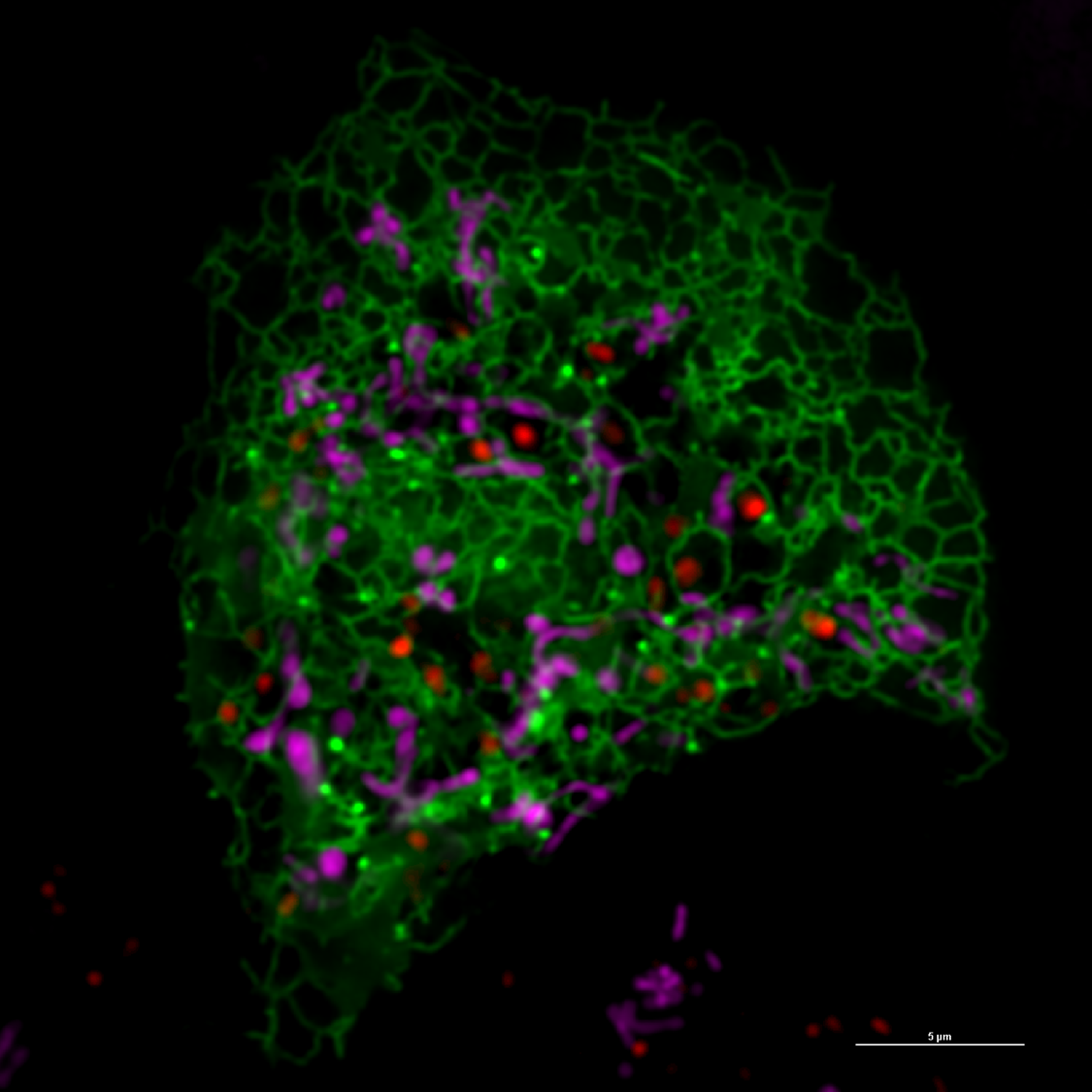 A super resolution image captured in Diao’s lab shows the endoplasmic reticulum in green, lysosomes in pink and mitochondria in red. Using the light-activated proteins, the mitochondria and lysosomes are brought together to conduct mitochondrial fission. Image Credit: Jiajie Diao.
A super resolution image captured in Diao’s lab shows the endoplasmic reticulum in green, lysosomes in pink and mitochondria in red. Using the light-activated proteins, the mitochondria and lysosomes are brought together to conduct mitochondrial fission. Image Credit: Jiajie Diao.
Research findings
The study focuses on the roles played by mitochondria, which are the cell’s “power plant” and primary energy source. Small, specialized structures called organelles carry out a variety of tasks inside cells.
To maintain equilibrium in healthy cells, hundreds of mitochondria are constantly joining together (a process known as fusion) and severing into smaller parts (a process known as fission), according to Jiajie Diao, PhD, one of the study’s authors.
However, this fission and fusion process is out of balance when mitochondria are not working properly.
Numerous mitochondrial disorders, including neurological conditions like dementia and some malignancies, can develop as a result of this imbalance.
According to Diao’s earlier studies, the lysosome, a different organelle found in cells, can contribute to the fission of mitochondria. The lysosome can behave like a pair of scissors and cut the mitochondria into smaller pieces when it comes in touch with them.
By placing lysosomes and mitochondria together inside of cells, the current study sought to accelerate the fission process. Optogenetics, a method that uses light to precisely control certain cell activities, was used to achieve this.
Many proteins in plants are light sensitive, informing plants whether it is day or night. Optogenetics borrows these light-sensitive proteins from plants and uses them in animal cells. By attaching such proteins to organelles, one can use light to control the interaction between them, such as mitochondria and lysosomes shown in this work.”
Kai Zhang PhD, Study Co-Author and Associate Professor, University of Illinois Urbana-Champaign
Kai Zhang developed the optogenetic tools for controlling mitochondria and lysosomes with blue light.
The scientists joined two distinct proteins to lysosomes and mitochondria in stem cells. The proteins spontaneously join to one another to generate a new protein when triggered by blue light, which also connects the mitochondria and lysosome. The lysosome can sever the mitochondria once they are combined, causing fission.
We found that it can recover the mitochondrial function. Some of the cells can even go back to normal. This proves that by just using some simple light stimulation we can at least partially recover the mitochondrial function of the cell.”
Jiajie Diao, Associate Professor, Department of Cancer Biology, College of Medicine, University of Cincinnati
Jiajie Diao is also a University of Cincinnati Cancer Center member.
According to Diao, this method may be particularly helpful for patients whose drastically enormous mitochondria need to be broken into smaller pieces in order for the cells to operate normally. The method could also be used to treat cancer cells by repeatedly cutting apart the mitochondria until they are no longer able to function.
Eventually the cancer cells will be killed because mitochondria is their energy. Without normal functional mitochondria, all of the cancer cells will be killed.”
Jiajie Diao, Associate Professor, Department of Cancer Biology, College of Medicine, University of Cincinnati
According to Diao, the fact that the proteins are light-activated enables a more specialized approach to particular cells. The approach does not cause the mitochondria of neighboring healthy cells to become out of balance because it only affects cells that are exposed to light.
Other methods for inducing mitochondrial fission are now available, but Diao says that the optogenetic approach is safer because it uses no chemicals or hazardous substances.
Diao adds, “What we have is actually the natural process, we’re just making it faster. So it’s not like a chemical or a therapy or a radiotherapy where you need to reduce the side effects.”
Next steps
When mitochondria are out of balance because they are too small and are not fusing together properly within cells, Diao said his team is already working on utilizing the same strategy to promote fusion.
Since a longer wavelength will be required to reach human tissue, additional research from Zhang’s lab will also focus on creating new optogenetic devices that use multiple colors of light, including green, red, and infrared.
Zhang explains, “We would like to further expand the toolbox by introducing multicolor optogenetic systems to give us multiple ways to control how organelles behave and interact. For instance, one color makes organelles come together, while the other color forces them apart. This way, we can precisely control their interactions.”
The team wants to build on the existing research using human stem cells to assess the technique’s effectiveness using animal models and, eventually, in humans through clinical trials. Diao said that other research teams are investigating the use of magnetic fields and acoustic vibrations in place of light to achieve comparable outcomes.
Source:
Journal reference:
Qiu, K., et al. (2022) Light-activated mitochondrial fission through optogenetic control of mitochondria-lysosome contacts. Nature Communications. doi.org/10.1038/s41467-022-31970-5.