Reviewed by Danielle Ellis, B.Sc.Sep 9 2022
Chlamydia, the most common bacterial infection associated with sexually transmitted diseases, hides inside human cells using a cloaking mechanism to avoid detection and destruction. However, scientists from Duke University have managed to get hold of the edge of that invisibility cloak and are now attempting to tear it apart.
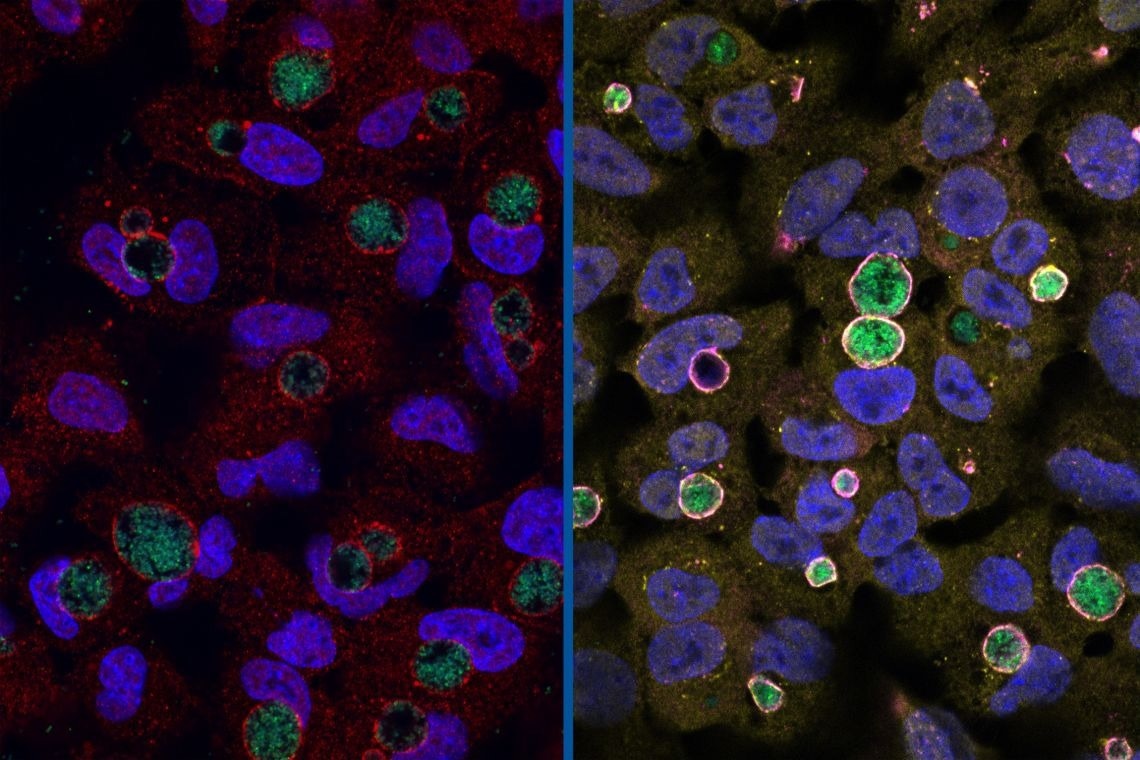 Left: Chlamydia (green) surrounded by the GarD protein (red) that cloaks it from detection. Right: Chlamydia with GarD knocked out (green) enveloped by antimicrobial ubiquitin proteins (yellow) and RNF213 (magenta). Image Credit: Stephen C. Walsh, Duke University
Left: Chlamydia (green) surrounded by the GarD protein (red) that cloaks it from detection. Right: Chlamydia with GarD knocked out (green) enveloped by antimicrobial ubiquitin proteins (yellow) and RNF213 (magenta). Image Credit: Stephen C. Walsh, Duke University
Many harmful bacteria, including Chlamydia, ensconce themselves in a portion of the cell’s membrane to enter the cell and proliferate peacefully.
This process creates an intracellular free-floating bubble known as a vacuole or, in the case of Chlamydia, an inclusion. The ability of Chlamydia’s cloak to circumvent the cell’s natural immunity appears to be particularly strong, enabling the infection to linger for months.
To understand how the cloaking functioned, a team from Duke led by graduate student Stephen Walsh and Jörn Coers, Ph.D., an associate professor of molecular genetics and microbiology in the Duke School of Medicine, carried out an investigation.
We knew there was the potential to kill Chlamydia, but when we did experiments with the human-adapted form, Chlamydia trachomatis, it was very good at growing in human cell cultures.”
Jörn Coers, PhD, Associate Professor, Molecular Genetics and Microbiology, Duke School of Medicine
Nothing happened even after the researchers administered an immune stimulant to notify the cell’s defense mechanisms that Chlamydia was there.
“We said, there’s the pathogen. Our defense system should see it. Why does it not see it?” exclaimed Coers.
To see how the cell’s immune system reacted to a disease that did not originate in humans, they repeated their studies using a mouse-adapted form of the Chlamydia bacteria in human cells.
“Humans, don’t get mouse Chlamydia because it evolved with mice, and human Chlamydia evolved with humans. So there’s this fine-tuned adaptation that the pathogen has undergone,” Coers said.
In human cells, the bacterial inclusion mouse variant was quickly recognized and marked for eradication.
Chlamydia trachomatis is so good at evading our human responses. It still causes an inflammatory disease, but it’s a very slow disease.”
Jörn Coers, PhD, Associate Professor, Molecular Genetics and Microbiology, Duke School of Medicine
The immune system and the pathogen have been engaged in an evolutionary battle for millions of years.
“Mouse- and human-adapted Chlamydia have a common ancestor. However, this common ancestor may go back as far as when humans and rodents split from each other. This is a long time for the bacteria to fine-tune their interactions with their host species,” Coers said.
In a broad genetic screening of Chlamydia, the scientist colleagues Raphael Valdivia and Robert Bastidas at Duke MGM discovered a protein called GarD (gamma resistance determinant), which looked to be preventing the host cell from designating a Chlamydia inclusion for immune system destruction.
The bacteria become vulnerable when their GarD genes were altered.
“GarD is the stealth factor,” Coers said.
GarD specifically prevents a large signaling protein known as RNF213 or mysterin from detecting tiny bacterial molecules that protrude from the inclusion’s outer shell.
“RNF213 is basically the eyes of the immune system,” Coers said. Mysterin is thus rendered blind, and the signal for immune flagging and destruction is never initiated.
These tiny, membrane-covered vacuoles, which are abundant inside cells, are mostly allies, but some, like the Chlamydia inclusion, can pose a threat.
“There are so many different types of membranes and vacuoles that live inside a cell,” Coers said.
“How is the immune system able to find the rare vacuole that contains a pathogen? In the case of Chlamydia, we really do not have the answer to that question. But whatever it is, we believe this enzyme (mysterin) is seeing it.”
Sadly, Coers stated, that is the extent of this story’s development at this time. This is a fantastic new understanding of a pernicious virus, but we are still some distance from a cure. Scientists still need to determine how mysterin initially detects those bacterial compounds and how GarD renders mysterin blind.
“If you could find a way to neutralize GarD, then you would shine a spotlight on Chlamydia for our immune system to see. There’s then the potential for the pathogen to be cleared before it can cause disease,” Walsh said.
The paper also includes inputs from Dennis Ko, Ph.D., and So Young Kim, Ph.D., both professors at Duke MGM, as well as a number of other graduate students and post-docs from the Coers and Ko labs. These authors are in addition to Coers, Walsh, Valdivia, and Bastidas.
A new Chlamydia infection affects 200,000 Americans each year, and it is spread through sexual contact and frequently goes undetected for months or even years.
An untreated infection can eventually result in female infertility, pelvic inflammatory disease, and ectopic pregnancies.
Young women should get a chlamydia test every year, according to the US Centers for Disease Control.
Source:
Journal reference:
Walsh, S., et al. (2022) The Bacterial Effector GarD Shields Chlamydia Trachomatis Inclusions from RNF213-Mediated Ubiquitylation and Destruction. Cell Host & Microbe. doi.org/10.1016/j.chom.2022.08.008.