Reviewed by Danielle Ellis, B.Sc.Sep 12 2022
The immune system is prepared to fight off internal threats like cancer in addition to outside invaders like viruses, bacteria, and parasites. However, cancers routinely avoid detection and outsmart the immune system’s defenses.
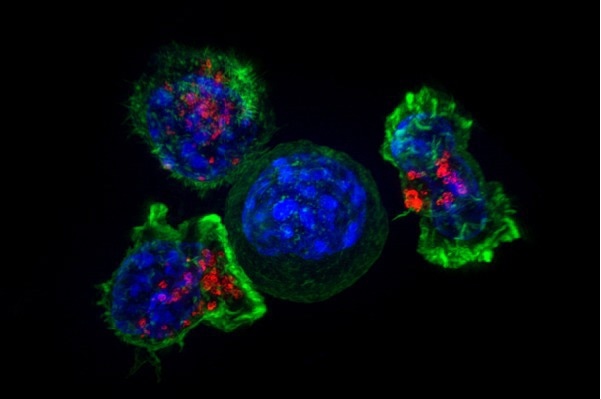 T cells surround a cancer cell to finish it off, but such interactions do not always end with the T cells victorious. Researchers from Penn detail how cancer cells can prompt T cells to ingest bits of cancer cell membrane, a process known as trogocytosis. The cancer may evade immune detection as a result. Image Credit: Alex Ritter, Jennifer Lippincott Schwartz and Gillian Griffiths, National Institutes of Health.
T cells surround a cancer cell to finish it off, but such interactions do not always end with the T cells victorious. Researchers from Penn detail how cancer cells can prompt T cells to ingest bits of cancer cell membrane, a process known as trogocytosis. The cancer may evade immune detection as a result. Image Credit: Alex Ritter, Jennifer Lippincott Schwartz and Gillian Griffiths, National Institutes of Health.
According to a recent study conducted by Serge Y. Fuchs of the School of Veterinary Medicine, cancers can avoid both the immune system and cancer treatments that depend on it, including CAR T cells that have been genetically altered.
When T cells engage with cancer cells, they can occasionally “nibble” a piece of the cancer cell membrane. Their study, which was published in the journal Cell Metabolism, disclosed how tumor-derived factors stimulate trogocytosis—a process derived from the Greek word trogo, which suggests “to gnaw” or “to chew.”
The T cells may start expressing that antigen on their own cell surface, making it seem to other T cells like a cancer cell if that membrane segment contains an antigen, a chemical particular to the tumor.
Trogocytosis can impact both the patient’s own T cells and those that have been altered to become CAR T cells, a treatment in which the patient’s T cells are genetically altered to target cancer cells selectively, cultured in a lab, and then returned to the patient.
Trogocytosis can lead to three different things, and all three are bad for a person with a tumor. First of all, the tumor cell did not get killed and has lost an antigen, which may mean that even if another, better equipped T cell comes along, it will not recognize it, giving cancer cells a window of opportunity to grow unchecked.”
Serge Y. Fuchs, Study Senior Author, School of Veterinary Medicine, University of Pennsylvania
Serge Y. Fuchs adds, “The second problem is, for reasons we still don’t understand, once a T cell takes a piece of the tumor cell membrane, it becomes much less active. And the third problem is very ironic. Because now, a T cell that displays tumor antigen, this ‘sheep in wolf’s clothing,’ may then become victim to ‘fratricide,’ killed by another T cell.”
Overall, this has the effect of reducing the quantity and activity of killer T cells while increasing the chances for cancer cells to proliferate and avoid detection.
What we see is that only a small number of cells undergo trogocytosis, and then they disappear quickly because they’re killed. So we’re studying a vanishing act. It is hard to do—very expensive and very tedious—but it appears to be very important.”
Serge Y. Fuchs, Study Senior Author, School of Veterinary Medicine, University of Pennsylvania
Fuchs and colleagues have long been interested in how immune cell surface receptors direct anti-cancer immunity, and they have produced a number of findings demonstrating how cancers can regulate T cells to avoid being targeted and killed.
Tumor-derived factors, or the mixture of proteins, lipids, and other substances that cancer cells exude into the body, are of particular interest. The Penn scientists discovered that the T cells' capacity to combat cancer was compromised when these secretions were collected and exposed to the resultant solution.
Just exposing them to this tumor-conditioned media caused them to kill fewer cancer cells, trogocytose more, and get killed more.”
Serge Y. Fuchs, Study Senior Author, School of Veterinary Medicine, University of Pennsylvania
The Penn-led study locked down the process, demonstrating that T cells exposed to tumor-derived substances had a considerable reduction in levels of the gene CH25H. Trogocytosis was previously thought to have something to do with the ability of cancer to impede anti-cancer immunity.
This gene is known to affect the lipid membranes of cells and can prevent the fusion of two cell membranes, which is required for trogocytosis to take place. They were able to stop trogocytosis by reintroducing a CH25H metabolite.
Fuchs states, “That was an ‘aha’ moment.”
The researchers were able to find another contributor by further analyzing the pathway: the gene ATF3, which inhibits the function of CH25H. Trogocytosis was halted by the removal of AFT3, and the capacity of T cells to destroy tumor cells was recovered.
The new discoveries not only point to potential new cancer treatment targets, but they also might have immediate implications for CAR T therapy. Trogocytosis may reduce the efficacy of the modified T cells given by CAR T, so the researchers hypothesized that preventing it might enhance CAR T performance.
Fuchs details, “We figured, why don’t we use what is cleverly known as an ‘armored CAR’ approach, and co-express CH25H in the CAR T. This turned out to be more efficient than the old CAR T cells.”
Delivering CH25H-adorned CAR T cells did prolong the lives of cancer-stricken mice compared to CAR T cells without the armor.
Image Credit: SciePro/Shutterstock.com
Trogocytosis only involves a small proportion of T cells, but according to Fuchs, it may be an unappreciated mechanism in terms of autoimmunity, cancer immunity, and other processes. He and his colleagues plan to investigate the functions of ATF3, CH25H, and other molecules in trogocytosis in the future.
He also has high hopes for future researchers to continue these lines of inquiry, bringing the research’s conclusions closer to therapeutic application.
Fuchs concludes, “I can see this going into use in CAR T therapy quickly. It’s ready to play.”
Source:
Journal reference:
Lu, Z., et al. (2022) ATF3 and CH25H regulate effector trogocytosis and anti-tumor activities of endogenous and immunotherapeutic cytotoxic T lymphocytes. Cell Metabolism. doi.org/10.1016/j.cmet.2022.08.007.