Researchers have opened a new avenue for investigations of neurological development, disease, and therapies that cannot be undertaken in living people by employing stem cells to grow small brain-like structures in the lab. However, not all mini-brain organoids are made equal, and enabling them to perfectly match the human brain tissues they are mimicking has proven challenging.
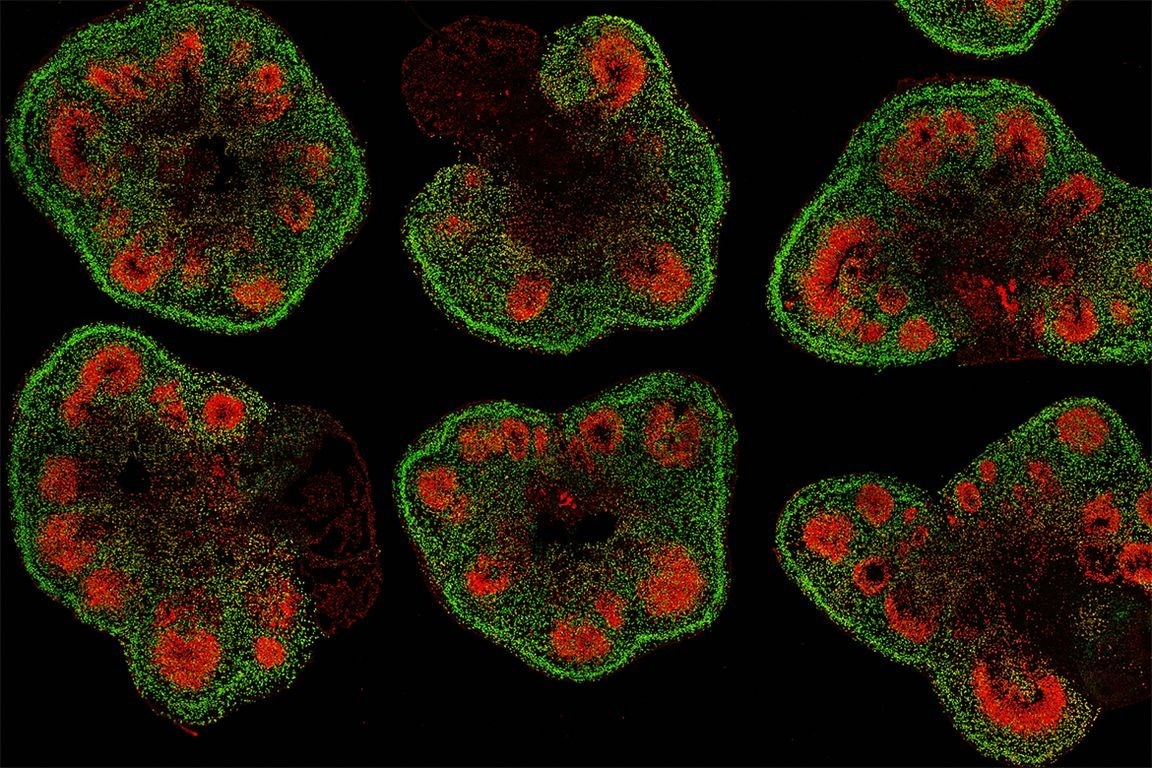 Slices of mini–brain organoids with neural stem cells (red) and cortical neurons (green). Image Credit: Hajime Ozaki, Watanabe lab/UCI.
Slices of mini–brain organoids with neural stem cells (red) and cortical neurons (green). Image Credit: Hajime Ozaki, Watanabe lab/UCI.
Right now, it’s like the Wild West because there is no standard method for generating mini–brain organoids. Every neuroscientist wants to make a brain organoid model of their favorite disease, and yet everyone’s organoids do not always look alike.”
Bennett Novitch, Study Senior Author and Member, Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, University of California, Los Angeles
Since there is no standard protocol for their creation and no quality-control criteria, organoids can differ from laboratory to laboratory—and even from batch to batch—which implies that a discovery made in one organoid may not hold true in another.
If my lab and another lab down the hall were to conduct drug screens using mini–brain organoid models of the same disorder, we could still get different results. We won’t know whose findings are correct because the differences we’re seeing could be reflections of how our models differ rather than reflections of the disease.”
Momoko Watanabe, Study First Author and Assistant Professor, Anatomy and Neurobiology, University of California Irvine
Novitch, Watanabe, and their co-workers suggested guidelines based on their findings that can assist scientists to overcome two main challenges preventing these organoids’ full potential: disparities in uniformity and structure. The findings were published in Stem Cell Reports.
Having organoids that precisely and steadily replicate the structure and cellular makeup of specific sections of the brain is particularly crucial when studying disorders like schizophrenia and autism spectrum disorder, in which affected people’s brains often appear structurally identical to neurotypical brains but display marked differences in function.
“We’ll never be able to identify the subtle differences in brain structure and function—things that are relevant for patients with neurological disorders—if our organoids have the wrong balance of cell types or grossly irregular structure,” added Novitch, who is also the director of the UCLA Brain Research Institute’s Integrated Center for Neural Repair.
Creating the best organoids: A question of maturity
To create mini-brain organoids, which can be 1 to 5 mm in diameter, researchers first reprogrammed human skin or blood cells to become induced pluripotent stem cells, which can develop into any cell type in the body.
They then directed these iPS cells to produce neural stem cells, which can generate nearly all cell types that exist in the brain. The neural stem cells can be induced to assemble into 3D organoids as they form. But why are some organoids more similar to the human brain than others?
The team worked with UCLA Broad Stem Cell Research Center pluripotency specialists Kathrin Plath and Amander Clark to discover the answer to this question. They found that an organoid’s quality is influenced by the developmental maturity of the stem cells from which it is developed, just as the freshness of the ingredients affects the flavor of a dish.
In human embryonic development, the nervous system is one of the first structures to form, so it makes sense that stem cells that are early in development are best at producing brain organoids.”
Momoko Watanabe, Study First Author and Assistant Professor, Anatomy and Neurobiology, University of California Irvine
Watanabe is also a member of the UCI Sue & Bill Gross Stem Cell Research Center.
The scientists then discovered that growing human stem cells in a dish with fibroblast feeders—mouse skin cells that provide crucial chemical signals and structural support for stem cells to expand and maintain their immaturity over time—was the most effective way to maintain these cells in an early developmental state suitable for organoid formation.
However, researchers found that utilizing mouse cells made organoids less suited for developing cellular therapies to repair diseased or damaged neural structures. Furthermore, these feeder-supported approaches are more time-consuming than the stem cell growth procedures usually used in labs.
The researchers next used RNA sequencing and computational analysis to try to identify genetic variations between stem cells that make effective organoids and those that do not. This permitted them to pinpoint four molecules, all of which were members of the transforming growth factor beta superfamily, that were responsible for preserving stem cells in a less-developed state.
Introducing these four chemicals to stem cells developing in a dish maintained them immature and allowed them to make high-quality, well-structured proteins.
Novitch concludes, “We found a way to have our cake and eat it, too. We have taken mouse cells out of the equation while retaining some of their benefits for organoid formation, bringing us closer to our goals of studying and developing treatments for complex neurological diseases.”
Source:
Journal reference:
Watanabe, M., et al. (2022) TGFβ superfamily signaling regulates the state of human stem cell pluripotency and capacity to create well-structured telencephalic organoids. Stem Cell Reports. doi.org/10.1016/j.stemcr.2022.08.013.